Root Hair Mutants of Barley
Kjeld C. Engvild and Søren K. Rasmussen
Plant Research Department, Risø National Laboratory,
DK-4000, Roskilde, Denmark
e-mail: kjeld.engvild(At)risoe.dk
Abstract
Almost sixty mutants with shortened or reduced root hairs or without root hairs at all have been isolated among 2000 M1 plant progenies (20.000 M2 seeds) of the spring barley cultivar “Lux” after sodium azide mutagenesis. One group of 25 mutants show fairly stable phenotype while 33 lines have unstable, indistinct or segregating phenotypes. The mutants were selected after 3 to 4 days growth in tap water on black filter paper. Root hair mutants were quite common, although not as common as chlorophyll mutants. Some of the unstable phenotypes may reflect responses to gases like ethylene or CO2. Shortened root hairs were sometimes a pleiotropic effect of other mutations, such as dwarf or chlorophyll mutants.
Introduction
Root hairs are small, tubular, 1-2 mm long extensions of single epidermal cells of the root surface. Their primary function seems to be to extend the surface of the root, thereby improving access to strongly bound nutrients like phosphorus, iron, zink, silicon and other micronutrients (Peterson and Farquhar 1996, Bibikova and Gilroy 2003). Root hair variation was first investigated in clover by Caradus (1979). There is one tomato mutant “cottony root” with longer root hairs than normal (Hochmuth et al. 1985). Root hair mutants have been investigated extensively in Arabidopsis, where a large collection of mutants is available (Grierson et al. 2001, Schiefelbein and Somerville 1990, Schiefelbein 2000). Root hair mutants have been studied in barley by Gahoonia et al. (2001) and by Szarejko et al. (2003). Root hairs are not absolutely necessary for plant growth, because root hairless mutants of barley, rice and maize grow quite well, if nutrients are readily available (Wen and Schnable 1996, Ma et al. 2001, Gahoonia et al 2001).At present about 40 genes are known to be involved in Arabidopsis root hair formation (Grierson et al. 2001, Parker et al. 2001).
Methods
Seed of “Lux” barley (Sejet Plant Breeding, Denmark) were soaked overnight in tap water, then treated with 1.5 millimolar sodium azide in 0.1 molar sodium phosphate buffer, pH 3, for 2.5 hours according to the IAEA manual on mutation breeding (2nd. Ed). After rinsing in tap water and air drying, the M1 seeds were sown in the field the same day. Spikes, 4-6 per M1 plant, were harvested.
9 or 16 seeds were germinated on 7 ´ 11 cm black filter paper in transparent polystyrene boxes 8 ´ 12 ´ 3 cm with almost tight-fitting lids. The germination took place in 5 ml of tap water at room temperature for 3 or 4 days. 0.5 mg Thiram decreased but did not eliminate fungal contamination. Scoring for root hair mutants was done directly through the transparent lid under a stereo microscope.
Results and discussion
The agar growth technique of isolating Arabidopsis root hair mutants did not work well for barley in our hands as there were many problems with infection. The chlorophyll mutation frequency of the mutagenized “Lux” material was 7 % of the M1 progeny and 0.9 % of the M2 seedlings, comparable to that obtained with other barley
cultivars from which low-phytate mutants were isolated (Rasmussen and Hatzack 1998).
The results show that root hair mutants can also be easily isolated in other plants than Arabidopsis. Most of the mutants are viable and some of them can undoubtedly be used as genetic markers, or in investigations of root hair physiology, root architecture, nutrient uptake, and the function and importance of mycorrhiza . Some of the mutants show unstable, variable or indistinct phenotypes. Some of these instabilities may be caused by lack of control of gases such as ethylene or CO2 which influence root hair formation (Pitts et al. 1998, Ohashi et al. 2003, Müller and Schmidt 2004). The large majority of mutants are recessive, judged by the segregation ratios of mutant/wild type among the M2 seeds. No crossing experiments have been done.
Due to other commitments the work on the root hair mutants has been discontinued at an early stage. Therefore the mutants have not been grown further than to M3 (M4 seeds). They need further purification of other unwanted mutations and more examination of phenotype stability. An important group of mutants without characterisation are the completely sterile root hair less which could presumably have defects in the tip growth mechanism common to pollen tube and root hair.
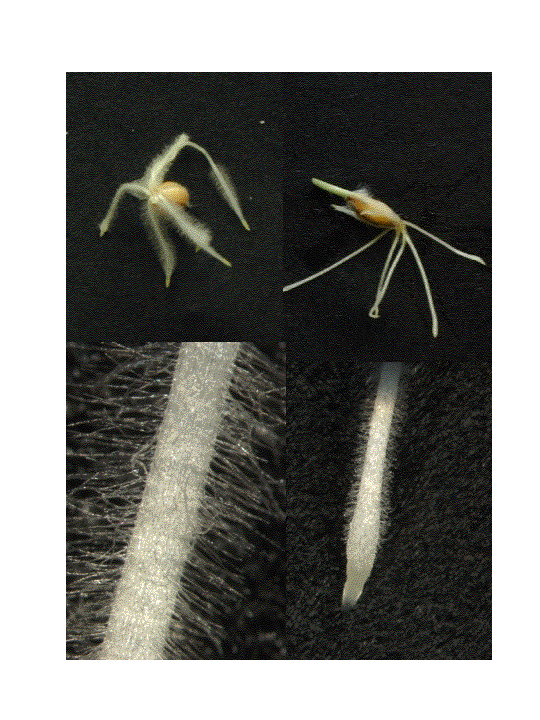
Figure 1. Wild
type “Lux” barley to the left, two mutants with very short root hairs to the
right
|
Table 1. Root hair mutants isolated among 2000 M1 progenies |
||
|
“Lux” Progeny No |
Phenotype |
Remarks |
|
|
|
|
|
76 |
Few root hairs |
|
|
460 |
Short |
|
|
465 |
Minus root hairs |
|
|
534 |
Minus root hairs |
|
|
681 |
Short |
|
|
754 |
Short |
|
|
837 |
Short |
|
|
846 |
Short, few |
|
|
919 |
Minus root hairs |
|
|
931 |
Short |
|
|
991 |
Short |
Swollen root tip |
|
1033 |
Minus root hairs |
|
|
1119 |
Short |
|
|
1205 |
Minus root hairs |
|
|
1209 |
Minus root hairs |
|
|
1210 |
Short |
|
|
1223 |
Short |
|
|
1339 |
Very short |
|
|
1400 |
Very short |
|
|
1533 |
Very short |
|
|
1537 |
Very Short |
|
|
1544 |
Very short |
|
|
1667 |
Minus root hairs |
|
|
1807 |
Short, few |
|
|
1920 |
Short |
|
|
Table 2. Putative mutants
that are not “well behaved”, either unstable, |
||
|
“Lux” progeny No |
Phenotype |
Remarks |
|
|
|
|
|
112 |
Short |
Poor germination |
|
160 |
Short |
Segregating tuft? |
|
194 |
Short |
Segregating? |
|
219 |
Short |
Segregating? |
|
226 |
Short, few |
Segregating? |
|
234 |
Tuft |
Indistinct |
|
244 |
Tuft |
Short root? |
|
250 |
Short |
Segregating? |
|
272 |
Short |
Indistinct |
|
337 |
Short |
Poor plants |
|
362 |
Short |
Segregating |
|
369 |
Short |
Infected |
|
430 |
Tuft |
Segregating? |
|
488 |
Short |
Indistinct |
|
522 |
Short |
Indistinct |
|
577 |
Minus root hairs |
Segregating short? |
|
635 |
Minus root hairs |
Segregating |
|
706 |
Minus root hairs |
Segregating |
|
741 |
Short |
Almost normal |
|
778 |
Short |
Sterility |
|
813 |
Short |
Poor plants |
|
840 |
Short |
|
|
923 |
Short, tuft |
Poor plants |
|
1261 |
Tuft |
Indistinct |
|
1263 |
Long? |
Indistinct |
|
1278 |
Short, few |
Poor plants |
|
1374 |
Short |
Segregating |
|
1450 |
Tuft? |
Indistinct |
|
1467 |
Tuft? |
Indistinct |
|
1505 |
Short |
Segregating |
|
1656 |
Short |
Segregating |
|
1707 |
Tuft |
Segregating |
|
1712 |
Tuft |
Indistinct |
|
1840 |
Short, tuft |
Indistinct |
|
1919 |
Short |
Segregating? |