

Genetic
diversity of Hordeum species assessed
by
Random
Amplified Polymorphic DNA markers
INRA, Laboratoire de Génétique et
d’Amélioration des Plantes,
Domaine de Brunehaut, F-80200
Estrées-Mons, France, laine@mons.inra.fr
The genus Hordeum contains more than 30 species
with a large natural area (Bothmer et al., 1995). Species are annual or
perennial and the ploidy level ranges from 2x,
4x to 6x with basic chromosome number x
= 7. Using morphological characters Bothmer et
al. (1995) classified Hordeum
species in four sections Anisolepis, Critesion, Hordeum and Stenostachys.
Botanical characters well defined the grouping of species in a same genus Hordeum, but the classification within Hordeum with genetic markers remains
subject to controversy (Marillia and Scoles, 1996 and the references therein).
Geographical distribution is also not always correlated with morphological
classification. The aim of this study was to assess genetic diversity with
molecular markers and investigate phylogenetic relationships in genus Hordeum.
Seeds from 42 species or subspecies of Hordeum and 4 varieties of cultivated barley (Table 1) were
germinated in Petri dishes for seven days. Thirty eight of the genotypes used
in this study were kindly provided by Professor Roland von Bothmer. Five young
seedlings were bulked for DNA extraction. PCR amplification was carried out
using a Genius thermocycler (Techne, Cambridge, UK). RAPD reactions were
realized with 10-base primers (AA19, AC09, G11, H14, I04, L04 and O19, Operon
Technologies Inc., Alameda, USA) according to Bahrman et al. (1999). Variable bands were scored and statistical analyses
were performed on a binary matrix using correspondence analysis. First, genetic
distances between all pairs of taxa and then, mean dissimilarity indices in
intra- and inter-section levels were computed (Bahrman et al. 1994).
A total of 77
variable bands were scored over all genotypes. High similarity levels were
found between H.euclaston and H. flexuosum (0.97), between all
cultivated varieties (0.89), between all pairs of H. bulbosum (0.85) and between H.vulgare
ssp spontaneum and H. vulgare ssp vulgare (0.80). In general intra-section distances were smaller
than inter-section ones. But, as indicated below, in two cases it was found to
be at the same level :
1 2 3 4
1- Anisolepis 0.36
2- Critesion 0.43 0.30
3- Hordeum 0.48 0.49 0.40
4- Stenostachys 0.39 0.37 0.46 0.36
All the distances between Hordeum sections were significatively
important, this may be because Hordeum species
have a large distribution in Eurasian region. It is important to note that
intra Critesion distance was smaller
than the other ones. This is probably due to the distribution of these species
in North and South America. All the inter-specific distances were larger than
intra-section ones. Correspondence analysis showed two main groups (Fig. 1).
These two groups contained Hordeum
species from American and Eurasian continents respectively, except H. secalinum, H. capense, H. bogdanii, H. brevisubulatum, H. roshevitzii, H. marinum which were misclassified.
Up to now, we gathered only partial data about
inter-specific distances with this limited set of RAPD markers. This study will
be continued using microsatellites loci and in the future we hope to bring more information about the Hordeum phylogeny.
Bahrman N., Zivy M., Damerval C. &
Baradat Ph. (1994) Organisation of the variability of abundant proteins in
seven geographical origins of maritime pine (Pinus pinaster Ait.). Theor. Appl. Genet. 88: 407-411.
Bahrman N., Le Gouis J., Hariri D., Guilbaud
L. & Jestin L. (1999) Genetic diversity of old French six-rowed winter
barley varieties assessed with molecular, biochemical and morphological markers
and its relation to BaMMV resistance. Heredity 83: 568-574.
Bothmer R. von, Jacobsen N., Baden C.,
Jorgensen R.B. & Linde-Laursen I. (1995) An ecogeographical study of the
genus Hordeum. International Plant
Genetic Resources Institute, Rome.
Marillia E. F. & Scoles G. J. (1996) The
use of RAPD markers in Hordeum phylogeny. Genome 39: 646-654.
Figure 1 – Correspondence analysis with
77 polymorphic RAPD bands, for abbreviation see Table 1.
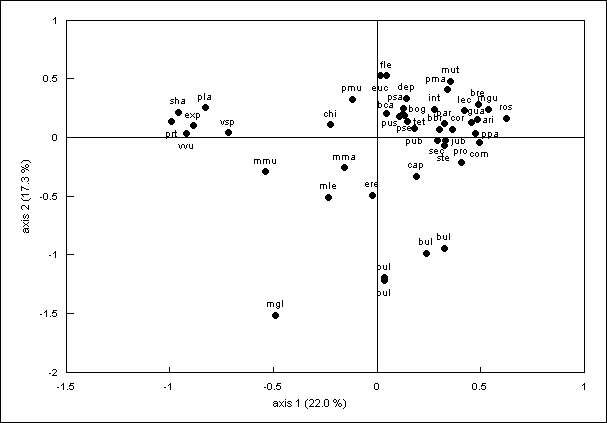
Table 1. Plant material and their
belonging to one of the four Hordeum
sections.
|
Section |
Species or subspecies |
Abbreviation
|
Distribution |
|
Anisolepis |
H. chilense |
chi |
South America
|
|
|
H. cordobense |
cor |
South America |
|
|
H. euclaston |
euc |
South America |
|
|
H. flexuosum |
fle |
South America |
|
|
H. intercedens |
int |
North America |
|
|
H. muticum |
mut |
South America |
|
|
H. pusillum |
pus |
North America |
|
|
H. stenostachys |
ste |
South America |
|
Critesion |
H. arizonicum |
ari |
North America |
|
|
H. comosum |
com |
South America |
|
|
H. jubatum |
jub |
North America, Asia |
|
|
H. lechleri |
lec |
South America |
|
|
H. procerum |
pro |
South America |
|
|
H. pubiflorum |
pub |
South America |
|
Hordeum |
H.bulbosum |
bul |
Eurasia, North Africa |
|
|
H.bulbosum |
bul |
Eurasia, North Africa |
|
|
H.bulbosum |
bul |
Eurasia, North Africa |
|
|
H.bulbosum |
bul |
Eurasia, North Africa |
|
|
H. murinum ssp glaucum |
mgl |
Eurasia, North Africa |
|
|
H. murinum ssp leporinum |
mle |
Eurasia, North Africa |
|
|
H. murinum ssp murinum |
mmu |
Eurasia, North Africa |
|
|
H. vulgare ssp spontaneum |
vsp |
Eurasia, North Africa |
|
|
H. vulgare ssp vulgare |
vvu |
|
|
|
Plaisant
|
pla |
|
|
|
Express |
exp |
|
|
|
Shannon |
sha |
|
|
|
Proctor |
prt |
|
|
Stenostachys |
H. bogdanii |
bog |
Asia |
|
|
H. brachyantherum ssp brachyantherum |
bbr |
North America |
|
|
H. brachyantherum ssp californicum |
bca |
North America |
|
|
H. brevisubulatum ssp brevisubulatum |
bre |
Asia |
|
|
H. capense |
cap |
South Africa |
|
|
H. depressum |
dep |
North America |
|
|
H. erectifolium |
ere |
South America |
|
|
H. guatemalense |
gua |
Central America |
|
|
H. marinum ssp marinum |
mma |
Eurasia, North Africa |
|
|
H. marinum ssp gussoneanum |
mgu |
Eurasia, North Africa |
|
|
H. parodii |
par |
South America |
|
|
H. patagonicum ssp magellanicum |
pma |
South America |
|
|
H. patagonicum ssp mustersii |
pmu |
South America |
|
|
H. patagonicum ssp patagonicum |
ppa |
South America |
|
|
H. patagonicum ssp santacrucense |
psa |
South America |
|
|
H. patagonicum ssp setifolium |
pse |
South America |
|
|
H. roshevitzii |
ros |
Asia |
|
|
H. secalinum |
sec |
Europe |
|
|
H.tetraploïdum |
tet |
South America |