

Mutations that result in a reduction of the amount of prolamine, which is deficient in the amino acid lysine, are of particular interest. They usually are accompanied by an increased amount of proteins that have essential amino acids.
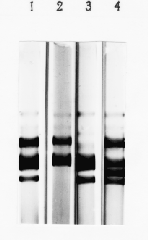
Three C-hordein deficient mutants derived from the cultivar Odessky 46 have been isolated: M564, M109, and M88. There is considerable variation in the numbers and positions of the components in the fractions from the different lines. Starch gel electrophoresis analysis (Pomortsev et al., 1985) of C-hordein fraction is shown in Fig. 1. These four lines differ in patterns of the C-hordein polypeptide. In the mutant M564, the C-hordein polypeptide pattern does not contain the prominant C-hordein polypeptide band. The C-hordein composition of M109 shows a reduction of upper polypeptide band, while in M88 one new band appears.
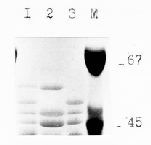
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli, 1970) shows the presence of a variable number of bands with relative molecular masses of between 50.0 kDa and 70.0 kDa (Faulks et al., 1981). The three lines were selected (Fig. 2) and there were clear differences in the numbers and mobilities of C-hordein bands separated by SDSPAGE. In M109 there is no polypeptide of 48.5 kDa, and M564 does not contain polypeptide with an MM of about 57.5 kDa.
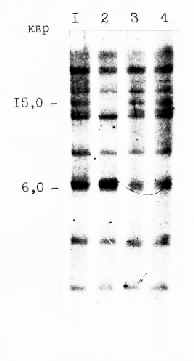
In order to identify fragments containing C-hordein genes in mutant barley endosperms, genomic DNA from wild type and mutant endosperms was isolated. The DNA was then digested with EcoRI restriction endonuclease. The resulting fragments were separated by electrophoresis, transferred to a nylon membrane, and probed with a 32P-labelled DNA related to coding region of C-hordein gene (Sayanova et al., 1993).
An autoradiograph of the Southern blot is shown in Fig. 3. A total of 11 different hybridizing fragments, with sizes ranging from less than 2 to about 20 kb, could be recognized. The hybridization pattern of DNA from M109 and M88 are identical to that of Odessky 46. In M564 the EcoRI fragment of about 15 kb is deleted. This DNA fragment may contain the gene for C-hordein polypeptide of 57.5 kDa which is absent in C-hordein fraction from M564. The failure of the M109 to produce C-hordein polypeptide of 48.5 kDa appears to be due to a point mutation too small to significantly change the restriction endonuclease fragment pattern of the C-hordein gene.
References:
Faulks, A. J., P. R. Shewry, and B. J. Miflin. 1981. The polymorphism and structural homology of storage polypeptides (hordein) coded by the Hor-2 locus in barley (Hordeum vulgare L.). Biochem. Genet. 19:841-858.
Laemmly, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685.
Pomortsev, A. A., V. P. Netsvetaev, and A. A. Sozinov. 1985. Hordein polymorphism of barley cultivar (Hordeum vulgare L.). Genetika 21:629-639.
Sayanova, O. V., S. L. Mekhedov, L. Zhelnin, T. A. Khokhlova, and E. V. Ananiev. 1993. Nucleotide sequence of C-hordein gene from barley. Genetika 29:15-23.