

One of the classic problems in barley nomenclature was the designation of mutations of a given phenotype that had been mapped, but not genetically tested with previously defined loci having the same phenotype. To overcome this problem, the comma comma system was introduced in BGN 11:1-18. For example, the male sterile mutant bk was mapped to chromosome 6 as msg,,bk before being genetically defined as an allele of a new gene, namely msg36 (Eslick et al., 1974; Ramage and Paluska, 1975; Franckowiak and Hockett, 1987). More recently a similar problem was encountered when preparing lists of the barley cDNA and genomic clones. The question was solved by appending the letter c or g, respectively, plus an arbitrary number of the basic symbol. Thus cDNA clones for the basic seed peroxidase (BP 1) and for a leaf peroxidase were given the temporary designations Prxcl and Prxc2 to distinguish them from the four genes in the literature Prxl, Prx2, Prx3, and Prx4, and to make clear that their relationship to these genes was unknown (Rasmussen et al., 1992; von Wettstein-Knowles, 1992). The letters c and g are thus analogues of the ,, notation. Subsequently Prxcl was mapped on chromosome 3 thereby intimating that it was a new peroxidase locus which was designated PrxS (Johansson et al., 1992).
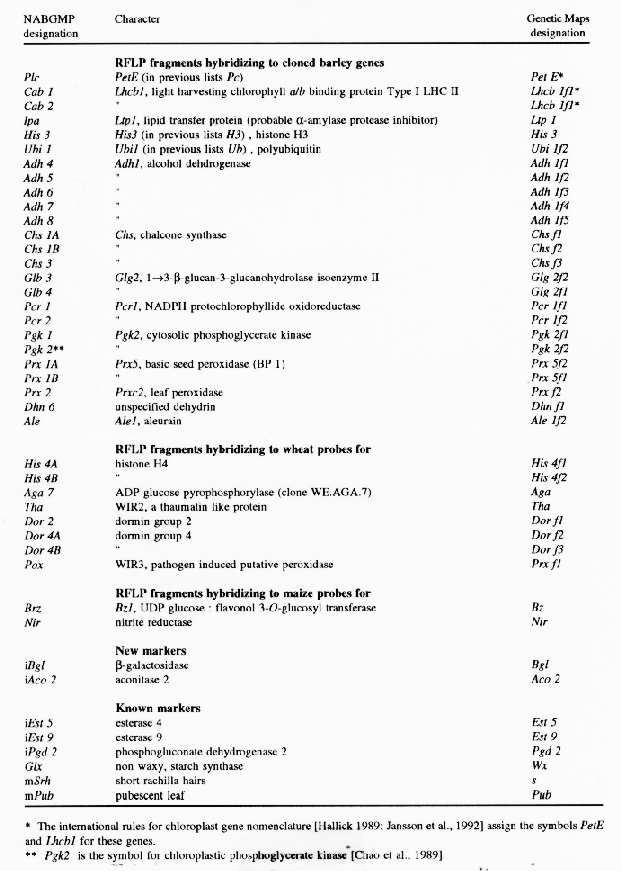
More recently the use of clones with known or presumedfunctions as probes in RFLP map construction has given rise once again to the same problem. For example, in the North American Barley Genome Mapping Project (NABGMP) report (Kleinhofs, 1992), a Chs probe for chalcone synthase hybridizes to three fragments at different loci. Using a letter f (fragment), in an analogous approach to the c, g, and ,, system described above, results in temporary designations for these three fragments as Chsfl, Chsf2, and Chsf3. Which of these three loci corresponds to the cloned Chs gene is an open question. When the PrxS clone, mentioned above, was used, it hybridized to two fragments, one each on chromosomes 3 and 1. These can be designated Prx5f1 and Prx5f2. Whether the fragment designated PrxSfl represents the same locus as that designated PrxS remains to be ascertained. Another example from the NABGMP report concerns a Pgk2 probe for cytosolic phosphoglycerate kinase, which had been mapped to chromosome 6 (Chao et al., 1989). This probe hybridized to two fragments, one each on chromosomes 4 and 1. These can be designated Pgk2fl and Pgk2f2 with the knowledge of both representing different loci than the chromosome 6 localized Pgk2 fragment.
The f as the ,, system of notation is independent of the source of the observations. For example, Alel, aleurain, was among the first barley genes isolated (Whittier et al., 1987), and was mapped to chromosome 1 using a probe for the 3' end of the gene (Li et al., 1991). An additional fragment strongly hybridizing to a probe for the 5' end of this gene was localized to chromosome 2, and shown to contain a 694 bp sequence very homologous to exon 3 plus intron 3 of Alel embedded in a sequence otherwise totally unrelated to that of Alel (Whittier et al., 1987; Li et al., 1991). Using the f nomenclature this chromosome 2 fragment becomes Alelfl. The NABGMP has mapped another fragment to chromosome 7, which can be temporarily designated Alelf2 until its relationship to the Alel locus on chromosome 1 designated Alel can be worked out.
In preparing the barley contribution to "Genetic Maps 1992" (S. J. O'Brien, ed., Cold Spring Harbor Laboratory Press), I have used the c, g, f, and ,, system to combine earlier defined barley genes with the new information coming from numerous laboratories. Table 1 was prepared to correlate the relationship between the collated information in Genetic Maps 1992 and theworking nomenclature of the NABGMP.
Chao, S., C. A. Raines, M. Longstaff, P. S. Sharp, M. D. Gale, and T. A. Dyer. 1989. Chromosomal location and copy number in wheat and some of its close relatives of genes for enzymes involved in photosynthesis. Mol. Gen. Genet. 218:323-430.
Eslick, R. F., R. T. Ramage, and D. R. Clark. 1974. Two genetic male steriles, msg6 and msg,,bk, assigned to chromosome 6. BGN 4:11-15.
Franckowiak, J. D., and E. A. Hockett. 1987. Allelism tests for the genetic male sterile msg,,bk. BGN 17:77-78.
Hallick, R. B. 1989. Proposals for the naming of chloroplast genes. II. Update to the nomenclature of genes for thylakoid membrane polypeptides. Plant Mol. Biol Rep. 7:266-275.
Jansson, S. et al. 1992. A nomenclature for the genes encoding the chlorophyll alb-binding proteins of higher plants. Plant Mol. Biol. Rep. 10:238-250.
Johansson, A., S. K. Rasmussen, J. E. Harthill, and K. G. Welinder. 1992. cNDA, amino acid and carbohydate sequence of barley seed-specific peroxidase BP 1. Plant Mol. Biol. 18:1151-1161.
Kleinhofs, A. 1992. The NABGMP mapping progress report, spring 1992. BGN 21:38-48.
Li, X.-Y., S. W. Rogers, and J. C. Rogers. 1991. A copy of exon 3-intron 3 from the barley aleurain gene is present on chromosome 2. Plant Mol. Biol. 17:509-512.
Ramage, R. T., and M. Paluska. 1975. Mapping chromosome 6. BGN 5:49-51.
Rasmussen, S. K., Johansson, A., Rasmussen, N. H., and Theilade, B. 1992. Molecular analysis and cloning of peroxidase genes. In: Biochemical, Molecular and Physiological Aspects of Plant Peroxidases. Lobarzewski, J., Greppin, H., Penel, C. and Gaspar, T. eds., Univ. of Geneva, Geneva, pp. 21-29.
Wettstein-Knowles, P. von. 1992. Cloned and mapped genes: current status. In: Barley . Genencs, Biochemistry, Molecular Biology and Biotechnology. Shewry, P. R., ed., CAB Intl., Oxon pp. 73-98.
Whittier, R. F., D. A. Dean, and J. C. Rogers. 1987. Nucleotide sequence analysis of alpha-amylase and thiol protease genes that are hormonally regulated in barley aleurone cells. Nucleic Acids Res. 15:2515-2535.