The 'Laevigatum' powdery mildew resistance has been used in European, particularly Danish spring barley (Munk et al., 1991), for nearly 30 years. Its durability has been tentatively explained by the effect of the relatively high infection type, 2-3 or 3 on seedlings (J0rgensen, 1983), or by the presence of more than one resistance gene (cf Wolfe, 1972). The present contribution confirms that the Laevigatum resistance is conferred by a single gene, Ml(La), that the powdery mildew fungus possesses a single virulence locus that matches gene Ml(La), and that gene Ml(La) is associated with long latent period, which is a component of partial resistance to barley leaf rust.
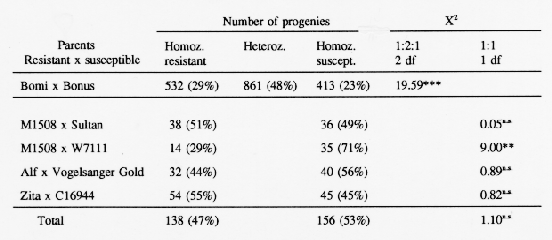
A large population of F2 plant progenies of 'Bomi' (Ml(La)) × 'Bonus' translocation lines (Table 1) showed a segregation ratio for mildew reaction close to a 1:2:1 ratio (Jensen et al., 1982). Four populations of chromosome doubled haploid (DH) lines were tested using appropriate powdery mildew isolates that had virulence corresponding to any powdery mildew resistance genes other than Ml(La) in the parents (cf Doll and Jensen, 1986). The parents with gene Ml(La) are 'M1508' and 'Alf', high-lysine and short straw mutants, respectively, and the cultivar 'Zita'. The segregations (Table 1) approached a 1:1 ratio. These data confirm that the Laevigatum resistance is conditioned by a single gene.
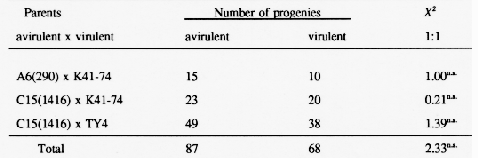
Four powdery mildew isolates, two Laevigatum avirulent and two virulent ones, were crossed and three progeny populations were tested for segregation in virulence. On Laevigatum resistant barleys (Table 2), the segregations were close to a 1:1 ratio indicating the presence of a single virulence locus with two alleles, V(La) and A(La).
These data strongly suggest that the Laevigatum host-pathogen interaction is conferred by a single gene pair in the host and one pair in the pathogen in accordance with the genefor-gene system.
Testing of the above mentioned mildew progeny isolates on eight barley lines possessing genes: Mla, Mla3, MlalO, Mlal2, Mlat, Mlra, Mlg, and Mlh showed 1:1 segregation ratios in each case. None of the eight virulence loci showed any sign of close (< 20% recombination) linkage with locus V(La).
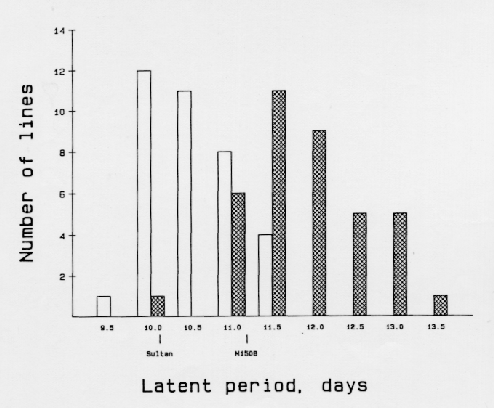
The DH-population from 'M1508' × 'Sultan' (Table 1) was tested for latent period in replicated field nurseries after inoculation with a leaf rust isolate by J. Parlevliet (pers. comm.) in The Netherlands (Figure 1). The average latent period of the 36 non-resistant lines was 10.5 days whereas that of the 38 Ml(La)-resistant lines was 11.9 days; this difference is highly significant. This shows that apparently one gene conferring extended latent period to leaf rust is located close to powdery mildew resistance gene Ml(La).
The cultivar Zita (Table 1) possesses a gene conferring resistance to barley leaf stripe that is linked to powdery mildew resistance gene Ml(La) with a recombination percentage of 20 (Haahr et al., 1989). Further, barley vaneties with the Laevigatum powdery mildew resistance gene are generally inferior in malting quality (cf Swanston, 1987). The Laevigatum powdery mildew resistance was transferred to European germplasm from a 'botanical' barley line Hordeum laevigatum (Dros, 1957). The data referred to and experiences accumulated by barley breeders suggest that a chromosome segment around 20 recombination units long has been transferred and maintained in many European spring barleys. It carries gene Ml(La) for powdery mildew resistance, a gene for leaf stripe resistance, at least one gene that prolong leaf rust latent period, and some that negatively affect malting quality. Recent data show that this chromosome segment is on barley chromosome 2 (H. Giese, J. Jensen, and H. P. Jensen, pers. comm.)
References:
Doll, H., and H. P. Jensen. 1986. Localization of powdery mildew resistance gene Mlra on barley chromosome 5. Hereditas 105:61-65.
Dros, J. 1957. The creation and maintenance of two spring barley varieties. Euphytica6:45-48.
Giese, H., A. G. Holm-Jensen, H. P. Jensen, and J. Jensen. 1992. Localization of the 'Laevigatum' powdery mildew resistance gene to barley chromosome 2 by the use of RFLP markers.n Theor. Appl. Genet. (submitted).
Haahr, V., J. P. Skou, and H. P. Jensen. 1989. Inheritance of resistance to barley leaf stripe (Drechslera graminea). Vortr. Pflanzenzüchtg. 15. Abstract no. 3-15. pp 2.
Jensen, H. P., J. H. Jørgensen, and J. Jensen. 1982. Attempts to locate powdery mildew resistance gene Ml(La) to a barley chromosome. BGN 12:65-68.
Jørgensen, J. H. 1983. Durability of powdery mildew resistance genes in Denmark 19631980. In: F. Lamberti, J. M. Waller and N. A. Van der Graff. Eds. Durable Resistance in Crops, Plenum Publ. Corp., London, p. 397-399.
Munk, L., H. P. Jensen, and J. H. Jørgensen. 1991. Virulence and disease severity of barley powdery mildew in Denmark 1974-1989. In J. Helms Jørgensen Ed. Integrated Control of Cereal Mildews: Virulence Patterns and Their Change. Risø National Laboratory, Roskilde, Denmark, p. 55-65.
Swanston, J. S. 1987. The consequences, for malting quality, of Hordeum laevigatum as a source of mildew resistance in barley breeding. Ann. Appl. Biol. 110:351-355.
Wolfe, M. S. 1972. The genetics of barley mildew. Rev. Pl. Path. 51:507-522.