

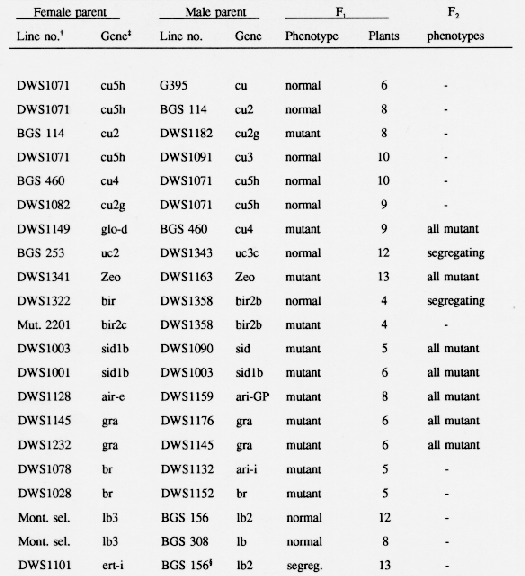
As entries in the collection of barley semidwarfs were backcrossed into a common genetic background, the cultivar Bowman, a few of the mutant phenotypes appeared to be similar to those conferred by previously described genes. Crosses were made among selected backcross derived lines to test for allelism, and F1 plants were grown in greenhouse pots. A few F2 progenies of 200 plants or more were grown in field plots to examine segregation ratios.
Curly leaf mutants
Two curly mutants, G395 (cu) isolated by Walker et al. (1963) and BGS114 (cu2) studied by Takahashi and Hayashi (1966), have strong coiling effects on roots, leaves, culms, and spikes. DWS1182, mutant number 2010 induced in Carina by H. Gaul (Franckowiak and Pecio, 1992), had similar phenotypic effects. Crosses made to test allelism (Table 1) demonstrated that DWS1182 contained an allele at the cu2 in chromosome 2. Two other alleles at the cu2 locus were described by Tsuchiya (1974b). The cu locus in chromosome 6 was not tested for allelism. Based on this limited data, the gene symbol cu2g is suggested for the mutant in DWS1182.
DWS1071 (SA6102-2-1-3-1), a sodium azide induced mutant isolated in 'Glenn' by Faue (1987), has coiled leaves and short culms. It was tested for allelism at the cu, cu2, cu3 (DWS1091, OUM 301), and cu4 (DWS1181, BGS 460) loci and was found to be nonallelic (Table 1). The cu3 (Hayashi et al., 1984) and cu4 (Tsuchiya, 1984) mutants originated in Japanese cultivars. The original lines had short culm and coiled leaves and the mutants were placed in the curly group. The phenotype of DWS1071 was classified into the curly group only when the less pronounced phenotypes of cu3 and cu4 in the Bowman genetic background were considered. Based on allelism tests and phenotypic similarities, gene symbols cuSh and locus symbol cuS are suggested for the dwarfing gene in DWS1071.
As the uz gene and genes for extreme early maturity were removed from the BGS460 (DWS1181) stock by backcrossing to Bowman, the major phenotypic effects remaining were a slight coiling of leaves, a slighdy twisted upper peduncle, and short plump seeds. The phenotype resembled that of DWS1149, glo-d obtained from G. Fischbeck. Hauser and Fischbeck (1980) associated glo-d (globosum-d) with chromosome 2. The allelism test (Table 1) demonstrated that cu4 and glo-d are alleles. This result agreed with the suggestion of Hwang and Tsuchiya (1988) that cu4 may be located in chromosome 2.
Uniculum mutants
Entry DWS1343 (Mut. 3170) was obtained from F. Scholz and was described as a simply inherited uniculm mutant (Scholz and Lehmann, 1958). DWS1343 and its backcross derived Bowman lines had malformed spikes and were uniculm when grown in the field. When grown in greenhouse pots, most plants produced three tillers. The allelism test with a uc2 stock demonstrated that the mutated gene in DWS1343 is not allelic to uc2 (Table 1). Hence the gene symbol uc3c and locus svmbol uc3 are suggested.
Dominant semidwarfs
Another mutant gene (Mut. 2657) isolated by Scholz and Lehmann (1958) was included in the collection of barley semidwarfs as DWS1341 because it caused a significant decrease in plant height. Scholz and Lehmann (1958) described the gene controlling this phenotype as an incomplete dominant. In homozygotes, outer glumes were larger than normal but not in heterozygotes. A gene having similar effects was found in multiple dominant marker stocks developed by R. I. Wolfe (Wolfe and Franckowiak, 1991). The cross between DWS1341 and Wolfe's Multiple Dominant Marker Stock (DWS1163) produced only semidwarf plants (Table 1). Since both genes have dominant phenotypic effects, a large F2 progeny (200 plants) was grown. All plants in the F2 plants had the mutant phenotype. Dr. U. Lundqvist suggested the name zeocrithon (little barley) and the gene symbol Zeo for the mutant gene found in DWS1163 and DWS1341. Correspondence regarding the origin of Wolfe's dominant semidwarf was lost; therefore, it is possible that Mut. 2657 was the original stock.
Branched inflorescence, rachilla
Several mutants causing abnorrnalities in spike morphology were included in the semidwarf collection. Mutant 270 (DW1322), which modifies rachilla development, was included among the laxatum mutants provided by H. E. B. Larsson. It was described as having a branched inflorescence, rachilla or compositum and the bir(com) gene (Larsson, 1985). After several backcrosses to Bowman, DWS 1322 derived lines had a phenotype similar to those of DWS1358, a freak from a collection grown at CIMMYT in Mexico, and Mut. 2201, an induced mutant isolated by Scholz and Lehmann (1958). Allelism tests demonstrated that DWS1358 was not allelic to the laxatum-270 (DWS 1322) but was allelic to Mut. 2201. Few F1 plants were exarnined because only terminal spikelets were easy to emasculate. The locus symbol bir2 (com2) and gene symbols bir2b for DWS1358 and bir2c for Mut. 2201 are suggested. Later, bir2 was found to be present in the short arm of chromosome 2 (Franckowiak, 1992) while Larsson (1985) reported bir is in chromosome 7.
Single elongated internode dwarfs
Hayashi et al. (1984) mapped the gene for a single elongated internode (sid), only the peduncle elongates, in chromosome 4. The stock obtained from T. Konishi was assigned the semidwarf collection number DWS1090 (OUX 052 R101). Mutants with one or two elongated internodes were isolated also by L. Lehmann in the cultivar Birgitta. The collection numbers assigned were DWS1001 and DWS1003 and the original numbers were 17:09:3 and 17:11:3, respectively. Later, DWS1003 was found to be a mixture of two semidwarfs having different phenotypes and plants with the sid phenotype proved to be allelic to DWS1001 (Table 1). When DWS1003 was crossed to DWS1090, the F1 and F2 plants had the mutant phenotype (Table 1). Although expressivity of the mutant in lines from DWS1001 derived was not as strong as that in derived lines of DWS1090, the mutants appeared to be alleles at the sid locus. The allele symbol sid1b is suggested for the mutant gene present in DWS1001.
The Golden Promise mutant
The Golden Promise semidwarf mutant induced in Maythorpe was entered into the semidwarf collection in several cultivars: Midas (DWS1161), Clansman (DWS1166), and Fleet (DWS1349). Backcross derived lines of Golden Promise (DWS1159) and the other cultivars had a similar phenotype, a brachytic type growth habit with short culms, spikes, and awns. Several of the breviaristatum (ari) mutants had a similar phenotype, also. Because the Golden Promise mutant was reported to be in chromosome 7, allelism to the ari-e mutant was tested first (Persson, 1969; Persson and Hagberg, 1969). Results showed ari-e1, obtained from U. Lundqvist and maintained as DWS1128, and the Golden Promise mutant are alleles (Table 1).
Granum mutants
The mutant gene gra (gran-a) was described by Häuser and Fischbeck (1976). The phenotype was characterized by premature spike emergence, shortened internodes, narrow leaves, numerous tillers, and thin seed. As entries in the semidwarf barley collection were backcrossed into Bowman, plants having a similar phenotype in two-rowed lines were observed in the progeny of DWS1176 (HE2816 from M. Vásá) and DWS1237 (OR-SS-2 from P. M. Hayes). DWS1176 contained a second semidwarf gene that produced slightly curly plants and a third gene for strong gene for photoperiod response, Ea. DWS1237 also had Ea for response to photoperiod, which reduced the height of plants grown in North Dakota by about one third. Allelism tests (Table 1) demonstrated that the gra gene of DWS1145 was allelic to one gene present in DWS1176 and in DWS1237. OR-SS-2 was selected from a cross to the Tokak mutant and reported to have one gene for reduced height (Sears et al., 1981).
Brachytic mutants
The original barley mutant described as brachytic was in chromosome 1 and is present in DWS1078 (BGS 001). Tsuchiya (1974) demonstrated that the breviaristatum mutant, which was assigned the gene symbol aff-i (DWS1132), is an allele at the br locus. Later, Szarejko and Maluszynski (1984) isolated another induced brachytic mutant (DWS1152, 648AK or 035AR), and showed it is an allele at the br locus. Since a relatively large number of entries in the semidwarf collection had a brachytic phenotype (Franckowiak and Pecio, 1992), allelism tests were conducted for these entries first. The results confirmed earlier reports regarding allelism of these brachytic mutants (Table 1).
Long basal rachis internode
Several mutants causing abnormal elongation of the first internode of the rachis have been described in the literature. The first was observed often in six-rowed cultivars originating in introductions from Manchuria (midwestern six-rowed barley).It was described as the lb gene in chromosome 7, and is present in BGS 308, Wis. Ped 38. Induced mutants at two different loci were isolated by (Kasha and Walker, 1960) and assigned the symbols Ib2 and Ib3. They were mapped in chromosomes 4 and 1, respectively.
The stock for Ib3 was obtained from T. Blake, Montana State University, Bozeman, MT, but it was labeled Ib2 or Ib3. Crosses to BGS 156 (Ib2) and BGS 308 showed that the stock from Montana did not contain an allele at the lb or Ib2 loci. Thus, I assumed that the Montana stock maintained by R. F. Eslick contained the Ib3 gene isolated by Kasha and Walker (1960).
Since the backcross derived lines having Ib2 appeared similar in phenotype to backcross derived lines of DWS 1101 (ert-i), crosses were made to test allelism. The Fl plants segregated for the Ib2 phenotype (semidwarf) and normal. This suggested that the ert-i locus described by Persson and Hagberg (1969) is the same as the Ib2 locus. If this result can be reconfirmed, the current linkage map of chromosome 4 contains two markers at different positions which are alleles.
References:
Faue, A. C. 1987. Chemical mutagenesis as a breeding tool for barley. M. S. Thesis, North Dakota State Univ.. Fargo, ND.
Franckowiak, J. D. 1992. Mapping a gene for photoperiod sensitivity in barley. Agron.Abstr. 1992:96.
Franckowiak, J. D., and A. Pecio. 1992. Coordinator's report: Semidwarf genes: A listing of genetic stocks. BGN 21:116-127.
Häuser, J., and G. Fischbeck. 1976. Untersuchungen zur Lokalisierung einger Matationen von Gerste (Hordeum sativun.). Z. Pflanzenzüchtg. 77:269-280.
Häuser, H., and G. Fischbeck. 1980. Genetic analysis of induced mutations. BGN 10:30-31.
Hayashi, J., T. Konishi, I. Moriya, and R. Takahashi. 1984. Inheritance and linkage studies in barley VI. Ten mutant genes located on chromosomes 1 to 7, except 5. Ber. Ohara Inst. landw. Biol., Okayarna Univ. 18:227-250.
Hwang, J. J., and T. Tsuchiya. 1988. Primary trisomic analysis of the gene cu4 for curly 4 (spiral) mutant in KM 118. BGN 18: 18-20.
Kasha, K. J., and G. W. R. Walker. 1960. Several recent barley mutants and their linkages. Can. J. Genet. Cytol. 2:397-415.
Larsson, H. E. B. 1985. Linkage studies with genetic markers and some laxatum barley mutants. Hereditas 103:269-279.
Persson, G. 1969. An attempt to find suitable genetic markers for dense ear loci in barley II.Hereditas 63:1-28.
Persson, G., and A. Hagberg. 1969. Induced variation in a quantitative character in barley. Morphology and cytogenetics of erectoides mutants. Hereditas 61:115-178.
Scholz, F., and C. O. Lehmann. 1958. Die Gaterslebens Mutanten der Saatgerste in Beziehung zur Formenmannigfaltig Keit der Art Hordeum vulgare L.s.l. I. Kulturpflanze 6: 123-166.
Sears, R. G., W. E. Kronstad, and R. J. Metzger. 1981. Inheritance of dwarf and semidwarf plant height in barley. Crop Sci. 21:828-833.
Szarejko, I., and M. Maluszynski. 1984. New brachytic mutant of spring barley variety Aramir. BGN 14:33-35.
Takahashi, R., and J. Hayashi. 1966. Inheritance and linkage studies in barley. II. Assignment of several new mutant genes to their respective linkage groups by the trisomic method of analysis. Ber. Ohara Inst. landw. Biol., Okayama Univ. 13:185-198.
Tsuchiya, T. 1974a. Allelic relationships of genes for short-awned mutants in barley. BGN4:80-81.
Tsuchiya, T. 1974b. Further results of allelism testing in barley. BGN 4:82-85.
Tsuchiya, T. 1984. Inheritance of
Tsuchiya, T. 1986. List of barley genetic stocks. BGN 16:81-121.
Walker, G. W. R., J. Dietrich, R. Miller, and K. Kasha. 1963. Recent barley mutants and their linkages. II. Genetic data for further mutants. Can. J. Genet. Cytol. 5:200-219.
Wolfe, R. I., and J. D. Franckowiak. 1991. Multiple dominant and recessive genetic marker stocks in spring barley. BGN 20:117-121.