

Barley researchers have identified and placed in a collection many stocks containing male sterility inducing genes (Hockett, 1984; 1989; Hockett and Reid, 1981). Allelism tests completed thus far indicate that mutations for male sterility occurred at 46 different loci (Hockett, 1991) and involve 89 of the 405 mutants included in the collection. This study was designed to associate several male-sterile genes with a specific chromosome so that they could be more useful as genetic markers.
Multiple genetic marker stocks developed by R. I. Wolfe (Wolfe and Franckowiak, 1991) were used as male parents in crosses to selected malesterile stocks (MSS) obtained from E. A. Hockett. Unless previous data indicated a chromosome association, male sterile plants from the MSS were crossed to the Multiple Recessive Marker Stock (Table 1). The Multiple Dominant Marker Stock and chromosome specific stocks were used for a few crosses. Crosses were made in greenhouses and the F1 plants were grown in field or greenhouse nurseries. F2 generation of each cross was grown in field nurseries at Fargo in 1986, 1987, 1988, or 1989. Seeds were space planted mechanically and thinned so that observations could be taken on individual plants. Notes were taken on the morohological markers expressed by each plant.
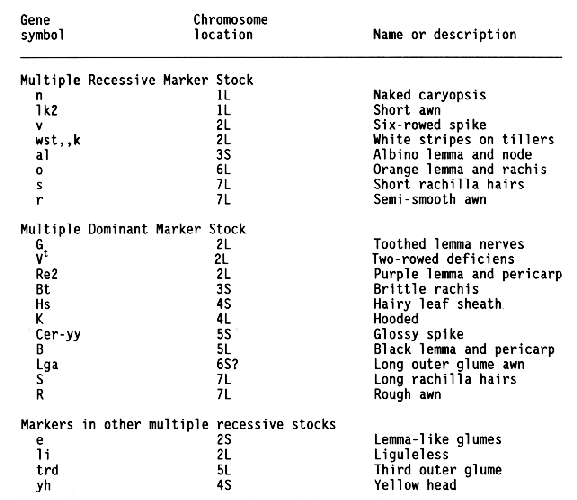
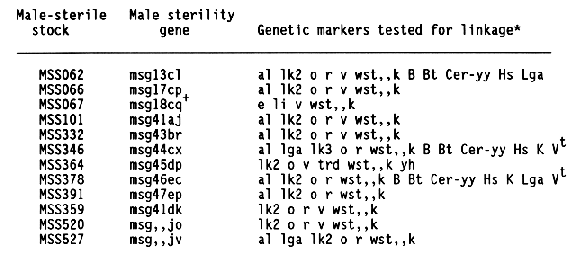
The data and chi-square values for goodness of fit to a 3:1 ratio, which indicated linkage between a male-sterile gene and a morphological marker, are summarized in Table 2. Linkage distances were estimated from F2 data using the product method (Immer and Henderson, 1943). Data for association between a male sterile gene and a marker that did not provide positive evidence for linkage are not shown. Male-sterile genes for which no evidence of linkage to a specific marker are listed in Table 3. Linkage data for msg8ch, msg11az, msg27ae, msg29a, and msg34av were reported earlier (Franckowiak, 1987).
For the male-sterile genes having completed allelism tests and for which in previous linkage information was not available, crosses to the Multiple Recessive Marker Stock provided some positive linkage information for 12 of 21 male-sterile genes. Subsequent crosses to the Multiple Dominant Marker Stock located 1 of 4 male-sterile genes, msg31d. Crosses to MSS lines for which allelism tests are not complete resulted in positive linkage data for 13 of 16 male-sterile genes. Since four of these malesterile genes showed linkage to lk2 locus on chromosome 1 and six to the al locus on chromosome 6, some of them are likely to be alleles to each other or previously mapped male-sterile genes.
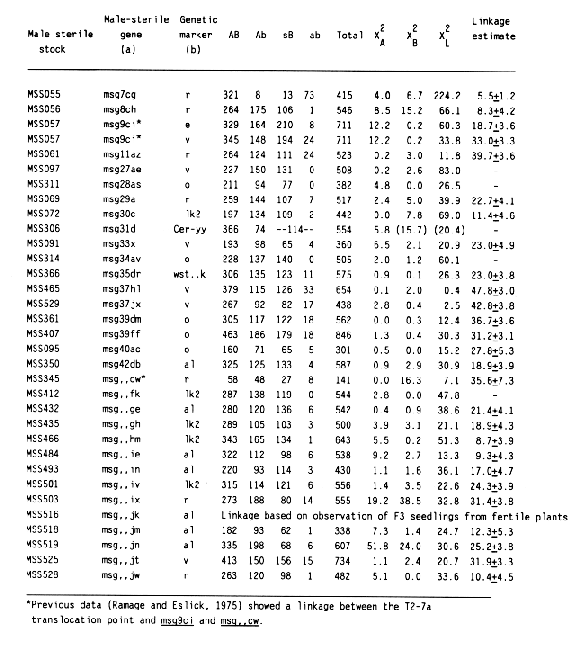
Some of the male-sterile and marker genes had segregation ratios that deviated significantly from 3:1 (Table 2). Reduced transmission of msg,,ix, msg,,jk, msg,,jm, and msg,,jn were reported also by Hockett (1984). In the cross to msg9ci, the frequency of male sterile plants was higher than expected. The reduced recovery of msg31d plants was probably caused by poor seedling vigor and loss of plants homozygous for this male-sterile gene and the associated dwarfing. More plants classified as having smooth or semismooth awns were observed than expected. This trend seems to be associated with two-rowed cultivars of European origin.
Some of these male-sterile genes were evaluated for linkage by Ramage and Eslick (1975) in crosses to translocation 2-7 (T2-7a), which has break points in the short awn of both chromosomes. Their linkage data and the data reported here can be used to better estimate the chromosome location of a few male-sterile genes. Hockett and Eslick (1971) reported that msg7 and msg8 were closely associated. After linkage between msg8ch and r loci was observed, msg7cg was crossed to a line having rough awns, R. Since R locus is over 70 map units from the centromere, the negative linkage reported by Ramage and Eslick (1975) would be expected. Similarily, msg,,cw should be in an intermediate position between the centromeres of chromosome 7 and the R locus. The data for msg11az and msg29a indicates a weak linkage to the r locus. If this data and the negative data obtained by Ramage and Eslick (1975) are accurate, then both loci are located near the end of chromosome 7L.
The linkage estimates for msg9ci indicate it is closer to the e locus than the v locus. Since these loci are about 23 units apart and msg9 was linked to T2-7a, the msg9 locus should be on the short arm of chromosome 2. Linkage to chromosome 2 markers was not observed, but Ramage and Eslick reported linkage of msg18ca and T2-7a. Thus, the msgl8 locus is more likely to be on chromosome 7.
No double recessive recombinants were obtained for five crosses; thus, linkage distances were not estimated. These crosses are a problem because either very close linkage, an inversion, or a translocation would give the same results. A translocation may be associated with msg34av, because F1 plants and some F2 plants have slightly reduced seed set, about 80%. Also, all the male sterile plants examined have long outer glume awns. The gene for long outer glume awn, Lga, has not associated with a specific chromosome; however, a gene producing a similar phenotype, E2, was reported to show linkage to the n locus on chromsome 1 (Robertson et al., 1965).
Ramage and Eslick (1975) reported that msg27ae segregated independently of T2-7a, but the result reported here indicate a strong association with the v locus. The T2-7a breakpoint maps near the e locus on the short arm of chromsome 2. Since the centromere is between e and v and the loci are about 23 map units apart, msg27ae should show linkage to T2-7a. Additional crosses with msg27ae will be made to evaluate this problem.
Phenotypes observed in the msg31d progeny could be classified into only three classes because plants homozygous for msg31d have glossy spikes. Since the genes were in the coupling phase in this cross, the observed deficiency of fertile plants with normal surface wax development on the spike indicates that msg31 is located on the short arm of chromosome 5.
Although only a tendency toward linkage to the v locus was observed in the msg37hl and msg37jx progenies, the data are presented in Table 2. Besides linkage, the observed deficiency of double recessive could be caused by misclassification of partially fertile plants with a six-rowed phenotype. Both recessive alleles at the msg37 locus show environmental induced variability in fertility level as does msg33x (Hockett and Eslick, 1971). Even though msg37 plants are completely male sterile in the winter greenhouse, seed set values over 70% were observed in some msg37 plants in segregating progenies.
The data presented in this paper demonstrates that multiple marker stocks are of value in locating some loci. But, their effectiveness for localization of specific genes may be poor. Because the chromosome specific stocks developed by R. I. Wolfe do not contain markers near the ends of all chromosome arms, they may not be as effective as trisomics for locating some genes.
References:
Franckowiak, J. D. 1987. Multiple recessive marker stocks for mapping male-sterile genes in barley. Barley Genetics V:209-212.
Hockett, E. A. 1984. The genetic male sterile barley collection. BGN 14:70-75.
Hockett, E. A. 1988. New mutants in the genetic male sterile barley collection. BGN 18:70-73.
Hockett, E. A. 1991. The genetic male sterile collection. Identification of eight new loci and allelism of 14 additional mutants. BGN 20:37-40.
Hockett, E. A., and R. F. Eslick. 1971. Genetic male-sterile genes useful in hybrid barley production. Barley Genetics II:298-307.
Hockett, E. A., and D. A. Reid. 1981. Spring and winter genetic malesterile barley stocks. Crop Sci. 21:655-659.
Immer, F. R., and M. T. Henderson. 1943. Linkage studies in barley. Genetics 28:419-440.
Ramage, R. T., and R. F. Eslick. 1975. Translocation linkage tests T2-7a x male sterile genes. BGN 5:46-48.
Robertson, D. W., G. A. Wiebe, R. G. Shands, and A. Hagberg. 1965. A summary of linkage studies in cultivated barley, Hordeum species: Supplement III, 1954-1963. Crop Sci. 5:33-43.
Wolfe, R. I., and J. D. Franckowiak. 1991. Multiple dominant and recessive marker stocks in spring barley. BGN 20:117-121.