SIBERIAN INSTITUTE OF PLANT PHYSIOLOGY AND BIOCHEMISTRY
Siberian Division of the Russian Academy of Sciences, Lermontov str., 132, Irkutsk-33, P.O Box 1243, Russian Federation, 664033.
A.K. Glyanko and G.G. Vasilieva.
Attempts to classify plant organisms have been made for many years (Macleod 1894). Presently, phytobiologists prefer the classification of vital strategies (functional and ecocentric) proposed by Ramenskii (1938) and Grime (1979, 1988). According to this classification, all the plant organisms fall into three categories: competitors (C), stress tolerators (S), and ruderals (R). C strategists are plants characterized by high competitiveness. They are usually dominant and act to edify plant communities. S strategists are capable of surviving in unfavorable conditions and are adapted to life in extreme conditions. R strategists are characterized by a high level of contribution to reproduction. They are plants of damaged habitats with short life cycles (annual plants). The C, S, and R strategies seldom occur in nature as pure types, but plants of mixed strategies frequently prevail. Grime and colleagues have now identified 19 strategy types and developed methods for their determination (Hodgson et al. 1999).
During selection for economically beneficial features, cultivated plants have to a large extent lost their competitive properties and stress tolerance and may be characterized as ruderal plants. The basic quality of these plants is their ability to quickly respond to improved growth conditions via increasing growth, development, and productivity. Because competitiveness is a function of the R and S strategies (Usmanov 1987), ruderality and stress-tolerance properties may be regarded as strategies crucial for ecological stability in an agroecosystem.
Spring wheat is characterized by a mixed strategy that in favorable conditions may show as R/SR, where R is the prevailing property. Under unfavorable conditions, wheat plants may demonstrate stress-tolerance traits by expressing genes of stability and redistributing energy and material input to increase stability at the expense of productivity. Under the long-term impact of an unfavorable factor (e.g., low positive temperature), wheat plants apparently implement an S/SR-type strategy, stress tolerance is a prevailing feature (in case the genotype has a sufficient, genetically conditioned potential for stress tolerance).
Depending on the variety, the proportion between ruderal and stress-tolerance properties is likely to fluctuate greatly. Indeed, within the huge amount of wheat varieties cultivated in various climatic zones of the world, there may be super ruderals, varieties with high production levels, and super stress-tolerators, varieties with high resistance to abiotic factors and relatively low productivity. The first type is comprised of varieties classified as intensive types, whose cultivation requires high energy input. Varieties of the second type are cultivated in areas with continental climates, for instance, in Siberia, North America, and Scandinavia.
Fig. 1 presents a scheme for the alterating plant properties (productivity and resistance) in a medium temperature environment and their connection with the type of vital strategy of wheat. At optimal temperatures for spring wheat cultivation (20-22 C), plants target growth to maximum yield to the highest extent (Rmax). In this case, plants are characterized by minimal resistance (Smin). This strategy type is R/SR. Wheat plants have the largest resistance and least productivity at suboptimal (5-10 C) and superoptimal (30-35 C) temperatures. This strategy type is S/SR.
These ideas may be useful for selection work aimed at cultivating spring wheat varieties with well-expressed competitiveness and tolerance to stress, which will facilitate the elimination of weeds and reduce the risk of large decreases in yield over years of unfavorable climate and other growing conditions.
References.
A.K. Glyanko and G.G. Vasilieva.
We are interested in plant nutrition with various forms of mineral nitrogen under conditions of soil drought. Resistance to drought stress in wheat is increased by using NH4^+^ compared to NO3^-^ (Spratt and Gasser 1970). The water potential in plants fed with NH4^+^ is reduced to a lesser extent than plants fed by nitrates in conditions of soil drought (Mihailovi et al. 1990). In optimal conditions, nitrates contribute to a higher amount of water in plant tissues than NH4^+^ (Kirkby and Mengel 1967; Krastina and Loseva 1975). We wanted to investigate the impact of various forms and doses of mineral nitrogen on the growth and certain physiological parameters of spring wheat cultivar Skala at the initial period of ontogenesis depending on the amount of soil moisture.
Materials and methods. Enamel tanks filled with to a capacity of 4 kg with dry sandy soil were used for the tests. A nutrient mixture incorporated macro- and microelements including nitrogen in the form of (NH4)2SO4 or Ca(NO3)2·4H2O + KNO3 (Thomas et al. 1979). Nitrogen dose in either form in the soil was at 100, 400, and 800 mg/tank or 7.1, 28.5, and 57.1 mM, respectively. The plants were grown in a glass house until 4-5 leaves had developed. Different moisture regimes in the tanks were assured by watering at the amount of 25, 40, 60, 80, and 100 % of the total soil-water intake capacity (SWIC). In order to prevent nitrification, nitrapyrine at the amount of 1.5 % of nitrogen was added to the vessels with (NH4)SO4. Nitrates in plant tissues were determined by the method of Cataldo et al. (1975) and total reduced nitrogen by the micro-Kjedal method (Ermakov 1987). Plant samples were dried at 60°C to calculate the weight of the total water content in the plant tissues. The results are presented as the mean ± standard deviation.
Results and discussion. The impact of various degrees of soil moisture on water amount in the tissues and nitrates content in the plants. We found a distinct positive correlation between the increase in soil moisture (from 25 to 80 % of SWIC and water amount in the surface part of the plants (r = +0.94) and nitrate content in leaves and stalks (r = +0.97). This dependence was discovered with an NO3^-^ content in the soil equal to 400 mg/tank.
NO3^-^ dose impacts water amount in the plants. An increase of nitrate concentration in the soil (from 100 to 400 mg) with optimal soil moisture (60 %) increases the amount of water in the tissues of surface organs. Water in the tissues decreases with a dose of 800 mg. In drought conditions (25 % of SWIC), water in the surface organs is half that for doses of 400 and 800 mg compared to those under optimal amounts of water (60 % of SWIC). With both soil moisture levels, a nitrogen dose of 800 mg produces a negative impact on the water amount in the tissues. Therefore, both low (100 mg) and high (800 mg) doses of NO3^-^ in the soil reduce water in plant tissue. With the optimal water amounts of 60 % of SWIC, the highest water amount in plant tissues is observed with 400 mg NO3^-^.
NH4^+^ doses impact the amount of water in the plants. We observed that the amount of water in tissues of plants with NH4^+^ nutrition is lower than plants utilizing nitrates and the amount of water in the tissues depends less on nitrogen dose with both optimal water amount and its deficit. Soil drought reduces the amount of water in the plants by 23-28 % under all nitrogen doses. In fact, nitrates may reduce the amount by a factor of two. The unequal influence of NO3^-^ and H4^+^ on water absorption by wheat is confirmed by calculating the amount of water in the plant surface part/gram of dry matter. This parameter in drought conditions decreases from 5.12 to 3.85 as level of NO3^-^ in the soil increases and increases with a rise in NH4^+^ (from 3.58 to 4.55).
The impact of different NO3^-^ amounts on nitrate content and their assimilation in surface organs. An increase in NO3^-^ in the soil during drought reduces the amount of nitrates released from the roots and available to surface organs and their assimilation by the plants. Thus, an increase of nitrate content in the soil from 100 to 400 or 800 mg decreases the level in the leaves by 17 and 27 % and in the stems by 21 and 40 %, respectively. At the same time, NO3^-^ assimilation, as evaluated by the content of total reduced nitrogen, drops in the leaves by 25 and 50 % and in the stems by 44 and 66 % at 400 and 800 mg, respectively. With NH4^+^ as an N source and insufficient water supply, the highest synthesis of nitric compounds in the leaves and stems is observed with nitrogen doses of 400 mg, over twice that at 100 mg and decreases by 25-33 % when nitrogen is increased to 800 mg.
Accumulation of dry matter by wheat seedlings. The greatest total dry matter accumulated by plants (at the four-leaf stage) in a moisture deficit (25 %) was observed at NO3^-^ levels in the soil of 100 mg (134.1 ± 5.8 mg/plant). Increasing NO3^-^ in the soil results in an abrupt drop of dry matter content of 90.3 ± 2.3 and 61.4 ± 1.6 mg/plant with the doses 400 and 800 mg, respectively. Using NH4^+^, the amount of dry matter accumulated by the plants is 112.8 ± 2.4, 134.2 ± 7.5, and 107.7 ± 4.1 mg/plant with nitrogen doses 100, 400, and 800 mg respectively. Thus, the highest degree of dry matter accumulation by wheat seedlings was observed with nitrate as the source of nitrogen and at a dose of 100 mg; with NH4^+^, the rate was 400 mg. Fluctuations in dry matter accumulation from different ammonium sources were expressed to a much lower degree than those with nitrate sources. A similar situation was found for water content in the tissues.
What accounts for the unequal influence of nitrates and ammonium on wheat growth in drought conditions ? We believe that the osmotic properties of nitrates and the different mechanisms for absorption and transportation of the nitrogen cation and anion to the surface organs may be the answer. Nitrates, being osmotically active ions (Veen and Kelinendorst 1986), may influence water absorption via a change in osmotic potential of the cells. Thus, an increase in NO3^-^ in the soil (from 100-400 mg) sharply increases water and nitrate uptake by the plants in the conditions of optimal water availability. This dependence is disturbed, however, in a water deficit and high NO3^-^.
Transfer of both NH4^+^ and NO3^-^ through the plasma membranes of the plant cells is by two transport systems. The first system functions at low nitrogen concentrations in the soil (up to 1 mM) (high-affinity transport system), the second system functions at high nitrogen concentrations (> 1 mM) (low-affinity transport system) (Goyal and Huffaker 1986; Cerezo et al. 2001). In our tests, we apparently observe the second transport system, which suppresses the sensitivity to nitrogen levels and the amount of water in the plants depending on the form of nitrogen. The majority of nitrates absorbed by wheat is known to be transported to the surface organs, where their assimilation (more energy-beneficial as compared to the roots) takes place (Lips 1997). Extreme factors (drought, salt buildup, or low temperature) inhibit nitrate transport to the surface organs (Glyanko 1995; Lips 1997). Because of these factors and as a consequence to nitrate-reductase inactivation in the roots during drought (Larsson et al. 1989; Il'chykov and Scher 1991), NO3^-^ accumulates in the roots blocking synthesis of organic N-containing compounds required for plant growth.
Incorporation of absorbed NH4^+^ in metabolism is known to take place completely in the roots. Thus, amides (glutamine and asparagine) are largely synthesized, and a portion is used for the root growth another portion is transported to the surface organs. NH4^+^ assimilation in the roots is accompanied by abscisic acid synthesis (Lips 1997), which is transferred along xylem to the surface organs together with amides, where it can influence transpiration reduction via influence on stomata functions (Farkhutdinov et al. 1982). Soil drought may be assumed to produce lower (as compared to a nitrate nitrogen source) negative impact on NH4^+^ absorption and its incorporation into roots and the transfer of N-compounds to the surface plant parts. Evidently, this can account for the positive influence on plant growth of high NH4^+^ levels in the soil both with optimal and insufficient availability of water for the plant.
References.
A.V. Kolesnichenko, E.L. Tauson, V.V. Zykova, E.S. Klimenko, O.I. Grabelnych, and T.P. Pobezhimova.
Plants adapted to unfavorable temperatures by different biochemical mechanisms. In particular, the synthesis of some groups of stress proteins increases under the influence of low temperatures (Abromeit et al. 1992; Crosatti et al. 1994; Houde et al. 1992). A large number of these proteins are regulated on the transcriptional level, but some are known to be regulated on translational and even posttranslational levels. The posttranslational level of regulation for bacterial cold-shock proteins (CSPs) was established by Chapot-Chartier et al. (1997) Craig et al. (1998), and Fang et al. (1997). Of the stress proteins with specific function that are localized in mitochondria, such as alternative CN-resistant oxidase (AOX) (Vanlerberghe and McIntosh 1992, 1997; Umbach and Siedow 1993) and the plant-uncoupling, mitochondrial protein (PUMP) (Jezek et al. 2000) are regulated not only at transcriptional but also at posttranslational levels. Thus, regulation at translation and posttranslational levels is widespread among stress proteins with specific functions, such as those that are known to be activate during short-time stress (Ladomery 1997).
Previously, we established that the plant cold-stress protein CSP 310, which is found in cereals (Kolesnichenko et al. 1996), causes uncoupling of oxidation and phosphorylation only during cold stress when this protein rapidly increases in amount (Borovskii et al. 1999). Although this protein was found to be synthesized constituently in plant cells, it did not cause significant uncoupling of oxidation and phosphorylation in nonstressed plants (Kolesnichenko et al. 2001a). Upon further investigation by native electrophoresis gel and ethidium bromide staining, we showed that CSP 310 differs in stressed and nonstressed winter rye shoots. In nonstressed shoots, ethidium bromide stains a nucleic-acid (NA) ligand band; in stressed rye shoots, the band of this protein in unstained (Kolesnichenko et al. 2000). These two forms of CSP 310 were found to have different uncoupling activities. The stressed form strongly caused uncoupling in plant mitochondria; the constituently synthesized form lacked an uncoupling activity (Pobezhimova et al. 2001). This fact allows us to propose that the release of a nuclear-acid ligand from CSP 310 could be a mechanism of CSP 310 uncoupling action regulation (Kolesnichenko et al. 2001b). At the same time, the nature of NA ligand in CSP 310 was not established. The mechanism of ligand release from CSP 310 during cold stress was not established either. Thus, the aim of the present work is to determine the nature of this nuclear-acid ligand and if the binding of the NA ligand depends on the temperature and the nature of the ligand.
Materials and methods. Three-day-old etiolated shoots of the winter wheat cultivar Irkutskaya Ozimaya, the winter rye cultivar Chulpan, X Triticosecale, Elymus sibiricus, and maize cultivar VIR 32 were grown on moist paper at 26 C. CSP 310 from nonstressed and stressed seedling shoots was isolated as described previously (Kolesnichenko et al. 1996). Proteins, immunochemically related to winter rye, stress protein CSP 310 from nonstressed winter rye, winter wheat, Elymus, and maize seedling shoots were isolated using affinity chromatography on a column with BrCN-activated Sepharose with immobilized anti-CSP 310 antiserum as described previously (Kolesnichenko et al. 1999).
The isolation of the nuclear-acid ligand from purified CSP 310 and from proteins immunochemically related to stress protein CSP 310 was performed using the standard method of NA deproteinizing and isolation (Manniatis et al. 1982). To isolate the NA ligand, 1.6 mg of CSP 310 was dissolved in 500 ml of water and then 60 ml of 10X TE buffer (pH 8.0), 15 ml pronase E (20 mg/ml), and 35 ml of water were added to CSP 310 solution. Pronase E treatment was performed for 2 h at 37 C, after which the NA ligands were isolated by phenol-chloroform extraction and ethanol precipitation.
In experiments with incubation of CSP 310 with different NA yeast, HMW RNA (Serva) (10 mg/ml dissolved in 10 mM Tris-HCl, pH 8.0) and one-stranded DNA- oligonucleotide 5'-acactagcttacggtgatct, 20 b was used. The 10X incubation buffer was 10 mM Tris HCl (pH 8.0), 60 mM KCl, 5 mM MgCl2, 1 mM DTT, 0.1 mg/ml BSA, and 12 % glycerol in ddH2O. Experiments were performed in two variants; with the constituently synthesized and stress forms of CSP 310. In the first variant, the stressed form of CSP 310 (1.6 mg of protein in 1,000 ml) was incubated with a) 10X buffer (150 ml), HMW RNA (100 ml) or H2O (250 ml); and b) 10X buffer (150 ml), 50 pM osDNA (5 ml) H2O (345 ml). In the second variant, the constituently synthesized form of CSP 310 (1.7 mg of protein in 2,000 ml) was incubated with a) 10X buffer (250 ml), HMW RNA (100 ml) H2O (150 ml) or b) 10X buffer (250 ml), 50 pM osDNA (5 ml), and H2O (245 ml). In both variants, the incubation mixtures were divided in two parts and one part was incubated at 0 C for 2 h and the other part was incubated at 26 C for 2 h. Thus, there were a total of eight variants of the experiment. After incubation, CSP 310 was precipitated from the incubation mixture by 50 % ammonium sulfate saturation (8 h at 4 C). The precipitated CSP 310 was collected by centrifugation (20,000 g, 20 min) and washed four times with 50 % ammonium sulfate solution. After washing, CSP 310 was dissolved in 1 ml 10 mM Tris-HCl (pH 8.0) and dialyzed against 10 mM Tris-HCl (pH 8.0) for 24 h at 4 C. The dialyzed CSP 310 preparations were hydrolyzed with pronase E as described above, and the NA ligands obtained were analyzed by electrophoresis in 1.5 % agarose gels (Manniatis et al. 1982). NA ligands were visualized by ethidium bromide staining.
Results and discussion. We first tried to isolate the NA ligand from preparations immunochemically related to CSP 310 proteins from some cereal species obtained by affinity chromatography from nonstressed-seedling shoots. In this experiment, we used the standard method of NA deproteinizing and isolation (Manniatis et al. 1982). The electrophoresis of nucleic-acid preparations obtained from winter rye and winter wheat immunochemically related to CSP 310 proteins show the presence of NA ligands (Fig. 2). At the same time, these ligands were not detected in immunochemical proteins related to CSP 310 from maize and Elymus (Fig. 2). DNAse treatment failed to eliminate bands of extracted NA on the gel (Fig. 3), so this NA ligand is not DNA. On the contrary, RNAse treatment eliminated bands of NA extracted on gel (Fig. 3). These results vindicate previous data on the presence of NA ligand in CSP 310 from winter rye (Kolesnichenko et al. 2000) and winter wheat (Kolesnichenko et al. 2001b). Indeed, CSP 310 was found among cytoplasmic proteins of winter rye and winter wheat, but was not found among maize and Elymus cytoplasmic proteins (Kolesnichenko et al. 1999). Based on these data, we concluded that in nonstressed winter rye and winter wheat shoots CSP 310 binds with an RNA ligand. The CSP 310-like proteins from maize and Elymus are not bound with any NA ligand.
The temperature of protein preincubation has no influence on
the presence of the RNA ligand in CSP 310. If CSP 310 extracted
from nonstressed shoots was bound with RNA independently from
the temperature of protein preincubation (26 or 0 C) (Fig. 4, lanes 2 and 3) in CSP 310 from stressed
(-1 C, 1 h) shoots, RNA was not detected (Fig. 4, lanes 4 and 5). Thus, we can distinguish
two forms of CSP 310, i.e., constituently synthesized (bound with
RNA) and stress (not bound with RNA) forms. Experiments with incubation
of stress form CSP 310 with different NA (HMW RNA and random osDNA
oligonucleotides) showed that the stress form of CSP 310 binds
the HMW RNA both at 0 and 26 C (Fig.
4, lanes 6 and 7). At the same time, the stress form did not
bind osDNA-oligonucleotides either at 0 or at 26 C (Fig. 4, lanes 8 and 9).
Our data show that constituently synthesized CSP 310 in nonstressed
winter cereals is a complex of protein with nuclear acid. This
NA ligand is RNA. At the same time, CSP 310 in stressed plants
is not so complex. The difference between the uncoupling actions
of these two forms of CSP 310 allows us to suppose that this RNA
ligand can be a regulator of its uncoupling activity (Pobezhimova
et al. 2001). The CSP 310 RNA ligand was found in cytoplasmic
proteins of winter rye and wheat immunochemically related to CSP
310, but not in CSP 310-like proteins of Elymus and maize.
These data confirm previous data about the presence of CSP 310
among cytoplasmic proteins of species investigated (Kolesnichenko
et al. 1999). Indeed, electrophoresis of native proteins followed
by Western blotting detected CSP 310 only among native proteins
of winter rye and wheat and only from CSP 310-like proteins of
these species were we able to isolate RNA. This RNA was isolated
from purified winter rye and Triticosecale CSP 310.
A study of the capacity of the stress form of CSP 310 to bind nuclear acids shows that CSP 310 binds HMW RNA but not osDNA oligonucleotides. These data allows us to suppose that CSP 310 can specifically bind RNA but not DNA and, therefore, can have RNA-binding sites in its structure. On the other hand, this RNA binding can take place because of conformational changes of native protein macromolecule. We note that the stress form of CSP 310 binds HMW RNA both at 0 and 26 C. This fact shows that the release of the RNA ligand during cold stress depends on the action of some mediators but not from the direct influence of low temperature. We can suppose that this mechanism of CSP 310-uncoupling activity regulation in winter rye and wheat can be a part of the integrated reaction of plant cells to low-temperature stress. In such cases, if the constituently synthesized form CSP 310 binds HMW RNA and can release it during low-temperature stress, it can participate in regulation of protein synthesis on translational level.
Based on the data obtained, we conclude that the constituently synthesized form of CSP 310 with low uncoupling activity binds with RNA ligand. During cold stress, this form of CSP 310 releases an RNA ligand and is transformed to the stress form of this protein with high uncoupling activity. This release does not depend on the direct action of the low temperature, because the stress form of CSP 310 can bind HMW RNA during incubation both at 26 and 0 C. CSP 310 can bind only HMW RNA but not osDNA oligonucleotides. CSP 310 can participate in regulation of protein synthesis on translational level.
Acknowledgments. The work was performed, in part, with the support of the Russian Foundation of Basic Research (project 01-04-48953).
References.
A.V. Kolesnichenko, O.I. Grabelnych, T.P. Pobezhimova, V.V. Tourchaninova, and V.K. Voinikov.
Cellular Ca^+2^ functions as an important cellular signal regulating most physiological processes in living organisms. This ion controls cell and mitochondrial reactions to various stress factors both in animals (Schefler 2000; McCormack et al. 1990; Kavanagh et al. 2000; Szabadkai et al. 2001) and plants (Khokhlova et al. 1993; Snedden and Fromm 1998) and controls such process as programmed cell death (Schefler 2000; Jones 2000; Kowaltowski 2000). Ca^+2^ has been established to have an important role in mediating the response of plant cells to different biotic and abiotic stimuli and trigger a large number of cellular processes that influence growth and the development (Jones and Mitchell 1989) that allow a plant to tolerate different stress conditions (Snedden and Fromm 1998). Mitochondria have a number of transport mechanisms by which they take up and release Ca^+2^ across their inner membrane and, therefore, participate in the regulation of a number of Ca^+2^-sensitive mechanisms in animal and plant cells (Schefler 2000; McCormack et al. 1990; Khokhlova et al. 1993; Snedden and Fromm 1998; Jones 2000; Kowaltowski 2000).
Recently, in cereals such as winter wheat and winter rye, a cold-stress protein CSP 310 that caused uncoupling of respiration and oxidative phosphorylation in cereal mitochondria during cold stress was found (Kolesnichenko et al. 1996; Voinikov et al. 1998). Studies of CSP 310 localization in vivo and in vitro showed increased amounts of this protein localized in mitochondria during cold stress (Kolesnichenko et al. 2000a and b). Previous researchers showed that Ca^+2^ ions regulated plant response to low-temperature stress (Khokhlova et al. 1993; Snedden and Fromm 1998). We were interested in determining if Ca^+2^ ions influence the CSP 310 function in cereal mitochondria.
One of the most important processes in which Ca^+2^ ion is
involved in animal cells is apoptosis (Petit et al. 1997). During
this process, mitochondria take up cytoplasmic Ca^+2^, which causes
PTP opening, collapse of mitochondrial membrane potential, release
of cytochrome c followed by activation of caspases, DNA fragmentation,
and, as a result, cell death (Petit et al. 1997; Smaili et al.
2000). This process in animal cells now is well studied. On the
other hand, programmed cell death is known to be an important
and integral part of the development of different plant tissues
such as endosperm (Young and Gallie 2000), aleurone (Fath et al.
2000), and tracheary elements (Fukuda 2000). Unlike animals, however,
programmed cell death in plants does not follow the apoptotic
way with nuclear condensation, cytoplasmic blebbing, and the involvement
of a macrophage to remove the corpse (Jones 2000). Though there
are a lot of data on the participation of reactive oxygen species
in both plant and animal PCD (Jabs 1999; Shirasu and Schulze-Lefert
2000), the main features of programmed cell death in plants are
a high degree of vacuolarization and an abrupt loss of plasma
membrane integrity, the activation of different nucleases and
proteases, and the loss of organelles as a result of cellular
autolysis (Jones 2000). Thus, programmed cell death in plants
is programmed autolysis (Fath et al. 2000) or in some cases it
may be programmed oncolysis (Jones 2000). Nevertheless, there
are data on the participation of cytosolic Ca^+2^ in programmed
cell death of many plant tissues (Groover and Jones 1999; Levine
et al. 1996; He et al. 1996).
The addition of CSP 310 to isolated winter wheat mitochondria
induces ascorbate-dependent and NADH-dependent lipid peroxidation
systems unlike other known uncoupling proteins (Zykova et al.
2000; Kolesnichenko et al. 2001a). However, we have shown that
the inhibition of CSP 310 by specific antiserum increases lipid
peroxidation in isolated mitochondria (Kolesnichenko et al. 2001b)
like other known uncoupling proteins (Kowaltowski 2000). Reactive
oxygen species (ROS) are known to form participates in PTP openings
(Petit et al. 1997; Smaili et al. 2000; Jabs 1999; Ridgley et
al. 1999) and, therefore, programmed cell death. This process
occurs more easily in mitochondria energized by complex I function.
Stress protein CSP 310 affects mainly complex I of the mitochondrial
respiratory chain (Fontaine et al. 1998). Because previously obtained
data showed some similarity between PTP opening and CSP 310 function
in cereal mitochondria, CSP 310 may influence the process of cytochrome
c release from winter wheat mitochondria. The aim of this present
work is to examine an influence of Ca^+2^ and CSP 310 on energetic
activity and cytochrome c release in cold-resistant winter wheat
mitochondria.
Materials and methods. Three-day-old etiolated shoots of the winter wheat cultivar Zalarinka were germinated on moist paper at 26 C. Mitochondria were extracted from control winter wheat shoots (germinated at 26 C) and stressed (-10 C for 1 h) winter wheat shoots by differential centrifugation as described previously (Davy de Virville et al. 1994). The isolated mitochondria were resuspended in 20 mM MOPS-KOH buffer (pH 7.4), 300 mM sucrose, 10 mM KCl, 5 mM EDTA, 1 mM MgCl2, 4 mM ATP, 6 mM ADP, 10 mM malate, and 10 mM glutamate.
In the first set of experiments, mitochondria isolated from control winter wheat shoots were divided into two parts and resuspended in the above mentioned media with or without an addition of CSP 310 (0.5 mg/1 mg of mitochondrial protein) and added in paleographic cell with or without an addition of different concentration of CaCl2 (1-50 µM). In the second set of experiments, mitochondria isolated from stressed winter wheat shoots were added in paleographic cell with or without an addition of different concentrations of CaCl2 (1-50 µM). The activity of mitochondria was analyzed 3-5 min after isolation.
Mitochondrial activity was recorded polarographically at 27°C using a closed-type platinum electrode in a 1.4-ml cell (Estabrook 1967). The reaction mixture contained 125 mM KCl, 18 mM KH2PO4, 1 mM MgCl2, and 5 mM EDTA, pH 7.4. An oxidative substrate of 10 mM malate in the presence of 10 mM glutamate was used. Polarograms were used to calculate the rates of phosphorylative respiration (state 3), nonphosphorylative respiration (state 4), respiration control by Chance-Williams, and the ADP:O ratio (Estabrook 1967). The concentrations of mitochondrial protein and CSP 310 were analyzed according to the method of Lowry et al. (1951).
To isolate CSP 310, 3-day-old etiolated shoots of winter rye germinated at 26 C and stressed at -1 C for 1 h were used. The isolation and purification of CSP 310 were performed in according to previously described methods (Kolesnichenko et al. 1996).
Release of cytochrome c from winter wheat mitochondria was measured by a spectrophotometer (SF-46, LOMO, USSR) according Moore and Proudlove (1983) at 550 nm after mitochondria precipitation. To measure cytochrome c release, the following medium was used: 300 mM sucrose, 40 µM MOPS (pH 7.4), 10 mM KCl, 2 mM EDTA, 1 mM MgCl2, 4 mM ATP, 6 mM ADP, 10 mM malate, and 10 mM glutamate (medium A). The reaction was initiated by adding 10 mM ascorbate and 0.1 mM TMPD. Cytochrome c amounts were calculated using the calibration curve 0.5-100 µM of cytochrome c as a standard.
Mitochondria isolated from control and stressed winter wheat shoots were incubated in either medium A + different CaCl2 concentrations or medium A + different CaCl2 concentrations + CSP 310 (1 mg/ml). Mitochondria were incubated at 0°C for 30 min and then were centrifuged at 20,000 X g for 3 min and the supernatant was used for cytochrome c measurement.
All the experiments were replicated six times. The data obtained were analyzed statistically by determining arithmetic means and standard errors.
Results and discussion. Ca^+2^ ions are known to be important for regulating most physiological processes and cells maintain a cytoplasmic concentration of this ion at very low levels. The cytosolic concentration of Ca^+2^ in mammals is about 7-10 M, and some researchers have used Ca^+2^ concentrations of 50-100 or 200 nM in their work (Smaili et al. 2000; Bowler and Fluhr 2000). Other data suggest that cytosolic Ca^+2^ concentration differs in plants and Ca^+2^ concentrations of 10 µM were used in the studies of Ca^+2^ influence on pea mitochondria (Vianello et al. 1995) and 0-30 µM in a study of Ca^+2^ influence on winter wheat mitochondria (Khokhlova et al. 1993). The concentration of Ca^+2^ in winter wheat roots in vivo was found to be about 1-2 µM/g f.w. (Minibayeva et al. 2000). In our study on the influence of Ca^+2^ on CSP 310 function in winter wheat mitochondria, we used Ca^+2^ concentrations 0-50 µM.
Mitochondria freshly isolated from winter wheat shoots had high energetic activity and a rather high degree of coupling of oxidation and phosphorylation. State-3 respiration without the addition of Ca^+2^ to the incubation medium was about 60 nM O2/min/mg of mitochondrial protein and state-4 respiration was about 22 nM O2/min/mg of mitochondrial protein at the same conditions (Fig. 5). The addition of different amounts of Ca^+2^ to the mitochondria-incubation media caused some changes in their activity. Low Ca^+2^ concentrations (1-5 µM) caused decreases in both state-3 and state-4 respiration to about 45-50 and 10-12 nM O2/min/ mg of mitochondrial protein, respectively (Fig. 5). The increase in Ca^+2^ concentration (5-20 mkM) caused an increase in state-4 respiration up to 20-25 nM O2/min/mg of mitochondrial protein, but state-3 respiration under these conditions was lower than in the variant without the addition of Ca^+2^ and was about 50-60 nM O2/min/mg of mitochondrial protein (Fig. 5). The presence of the acute maxima of state-3 and state-4 respiration at 10 mkM of Ca^+2^ is interesting. Furthermore, an increase in Ca^+2^ concentration (5-20 µM) caused decreases in both state-3 and state-4 respiration from about 10-40 nM O2/min/mg of mitochondrial protein, respectively (Fig. 5). Thus, different Ca^+2^ concentrations in mitochondrial incubation medium have diverse influences on mitochondrial energetic activity. If both low (1-5 µM) and high (25-50 µM) Ca^+2^ concentrations cause a decrease of state-3 and especially state-4 respiration, moderate Ca^+2^ concentrations did not have significant influence on their values.
The data obtained in our experiment on Ca^+2^ influence on mitochondria isolated from the super-cold-resistant, winter wheat Zalarinka differs from the data of Khokhlova and coworkers for mitochondria isolated from the medium-cold-resistant cultivar Mironovskaya 808 (Khokhlova et al.,1993). In their experiments without the addition of Ca^+2^, the values of both state-3 (~ 30 nanoatom O2/min/mg of protein) and state-4 (about 20 nanoatom O2/min/mg of protein) respiration obtained for mitochondria isolated from nonhardened seedling shoots in the winter were significantly lower than in our experiments (~ 60 and 22 nM O2/min/mg of protein for state-3 and state-4 respiration, respectively). In addition, in their experiments, peaks for both state-3 (up to 40 nanoatom O2/min/mg of protein) and state-4 (up to 25 nanoatom O2/min/mg of protein) respiration for mitochondria isolated in the winter from nonhardened wheat seedling shoots were detected. In our experiments, the maximum value for state-3 respiration in the variant without Ca^+2^ addition was observed. At 10 mkM Ca^+2^, state-4 respiration was only slightly higher than in variant without added Ca^+2^. On the other hand, at high Ca^+2^ concentrations both in our experiments and in experiments of Khokhlova with coworkers, the values of both state-3 and state-4 respiration were lower than without added Ca^+2^. These data allow us to propose that there is less affinity to Ca^+2^ in mitochondria isolated from super-cold-resistant winter wheat Zalarinka.
A study of CSP 310 influence on mitochondrial energetic activity was made after 1 h of mitochondria incubation, because the maximum increase in CSP 310 content in stressed winter rye shoots is detected during the first hour of cold stress (Borovskii et al. 1999). Although the physiological concentration of CSP 310 in cereals is about 0.25-1.0 mg/mg mitochondrial protein (Pobezhimova et al., 2001), a concentration of CSP 310 equal to 0.5 mg/mg of mitochondrial protein was used in all experiments.
Incubating isolated winter wheat mitochondria in vitro with CSP 310 previously was shown to cause an increase in nonphosphorylative respiration and the uncoupling of oxidative phosphorylation (Voinikov et al. 1998). A study of the influence of Ca^+2^ concentration in the mitochondrial-incubation medium showed its strong influence on CSP 310-dependent changes in state-4 respiration (Fig. 6). In the presence of both low (1-5 µM) and high (25-50 µM) Ca^+2^ concentrations in the medium, the addition of CSP 310 caused an increase in state-4 respiration. On the other hand, at moderate Ca^+2^ concentrations in the medium, the addition of CSP 310 caused state-4 respiration to decrease (Fig. 6).
The influence of Ca^+2^ on the CSP 310 effect on state-3 respiration in winter wheat mitochondria is similar but slightly different. If CSP 310 did not have any influence on state-3 respiration in the absence of Ca^+2^ or at concentrations in the range of 15-20 µM and with both low (1-5 mkM) and high (25-50 mkM) Ca^+2^ concentrations in mitochondrial-incubation medium, the addition of CSP 310 would cause an increase in state-3 respiration. At moderate Ca^+2^ concentrations in the medium, the addition of CSP 310 caused the decrease of state 3 respiration (Fig. 7). Of interest is the fact that at 7 µM of Ca^+2^ in the mitochondria-incubation medium, values for state-3 respiration with and without the addition of CSP 310 were similar.
Comparing the influence of cold stress (-1 C, 1 h) and CSP 310 on the respiratory-control coefficient in winter wheat mitochondria shows that they are similar at low (1-5 µM) and high (50 µM) Ca^+2^ concentrations in the mitochondria-incubation medium (Fig. 8). Cold stress and the addition of CSP 310, however, had an opposite influence on the mitochondrial respiratory-control coefficient at moderate (5-25 µM) Ca^+2^ concentrations (Fig. 8). We note that cold stress had no influence on the respiratory-control coefficient at Ca^+2^ concentrations around 10 µM (Fig. 8). When we compare our data on RC coefficient in control and stressed shoots with the data of Khokhlova coworkers for winter wheat with medium levels of cold-resistance (Khokhlova et al. 1993), it is interesting to note that in our experiments, the curve for the control shoots was similar to the data obtained for mitochondria isolated from shoots hardened in summer conditions than from those of the control under winter conditions.
A study of the influence of CSP 310 on the ADP:O ratio in winter wheat mitochondria showed that it was similar to that of cold stress only at low Ca^+2^ concentrations in mitochondrial incubation medium (Fig. 9). At these Ca^+2^ concentrations, both the CSP 310 treatment and cold stress caused a decrease of the ADP:O ratio in winter wheat mitochondria. At other Ca^+2^ concentrations, the ADP:O ratio in the presence of CSP 310 in mitochondrial-incubation medium was equal to or even higher than that of in control mitochondria. A similar effect on ADP:O ratio at these Ca^+2^ concentrations (especially at 10 µM) had cold stress. At the same time, our cold stress experiments caused a 10 µM peak of ADP:O ratio above the theoretical maximum, which is similar to the data obtained for nonhardened, medium-resistant winter wheat (Khokhlova et al. 1993).
Taken together, the data obtained on the influence of Ca^+2^ on CSP 310 activity in winter wheat mitochondria shows that at low (0-5 µM) and high (50 µM) concentration, the influence of cold stress and CSP 310 treatment are similar; both cause a decrease in the respiratory-control coefficient, whereas at medium Ca^+2^ concentrations, their influence is contrary. We suspect that this fact is under the control of the cell on CSP 310 activity by Ca^+2^ concentration.
Because Ca^+2^ is implicated in the process of programmed cell death both in mammals (Smaili et al. 2000) and plants (Fath et al. 2000), and a main feature of this process is Ca^+2^-dependent cytochrome c release from mitochondria to cytoplasm (Jones 2000; Smaili et al. 2000), we studied the influence of low-temperature stress and CSP 310 on cytochrome c release from mitochondria at different Ca^+2^ concentrations in the mitochondria-incubation medium.
First, the Ca^+2^ concentration in mitochondria incubation medium had a significant influence on cytochrome c release from winter wheat mitochondria (Fig. 10). Cytochrome c release from winter wheat mitochondria was very low without the addition of Ca^+2^; the addition of 1 µM of Ca^+2^ to mitochondria-incubation medium caused the release of about 1.8 µM of cytochrome c/mg of mitochondrial protein (Fig. 10). The increase of Ca^+2^ concentration to 5 µM caused a decrease of cytochrome c to 1 µM, and further increases in Ca^+2^ concentration to 10 µM and higher increased cytochrome c release to about 2 µM of cytochrome c/mg of mitochondrial protein (Fig. 10). Therefore, cytochrome c release in winter wheat mitochondria occurs in a Ca^+2^-dependent manner and, consequently, winter wheat mitochondria could participate in programmed cell death like in mammals.
Our data show that cold stress (-1 C, 1 h) has an influence on cytochrome c release from mitochondria at some low Ca^+2^ concentrations (0 and 5 µM) (Fig. 10). All Ca^+2^ concentrations (even without the addition of Ca^+2^), cytochrome c released from mitochondria was higher than 2 µM/mg of mitochondrial protein and at 10 µM of Ca^+2^ in mitochondria incubation-medium, cytochrome c release was nearly 3 µM. Therefore, cold stress causes similar effects on cytochrome c release in winter wheat mitochondria as in mammals, and we propose that winter wheat mitochondria participate in the process of programmed cell death caused by low-temperature stress.
CSP 310 dramatically influences the release of cytochrome c at low Ca^+2^ concentrations in winter wheat mitochondria (Fig. 10). The effect of the addition of CSP 310 to isolated winter wheat mitochondria on cytochrome c release at all Ca^+2^ concentrations was similar to the effect of cold stress. CSP 310 significantly increases cytochrome c release at low Ca^+2^ concentrations (0-10 µM) especially at 0 and 5 µM of Ca^+2^ in mitochondrial-incubation medium. From these data allow, we propose that CSP 310 participates in Ca^+2^-dependent cytochrome c release from winter wheat mitochondria during cold stress and, therefore, participates in programmed cell death.
The similarity between the influence of CSP 310 on mitochondrial energetic activity and cytochrome c release in winter wheat mitochondria is interesting. Indeed, the most pronounced uncoupling effect of CSP 310 on respiratory control coefficient and the release of cytochrome c at low Ca^+2^ concentrations (0-5 µM) in our experiments were detected. Because cytochrome c release from mitochondria depends on PTP opening (Petit et al. 1997; Jabs 1999; Smaili et al. 2000), we can speculate that CSP 310 at some Ca^+2^ concentrations can interact with the outer membrane voltage-dependent anion channel (VDAC) and cause PTP opening.
Based on these data, we conclude that Ca^+2^ influences CSP 310 function in winter wheat mitochondria. CSP 310 causes a decrease of respiratory control coefficient at Ca^+2^ concentrations about 1-5 µM and 50 µM and the increase of respiratory control ratio at Ca^+2^ concentrations about 10-15 µM. The influence of CSP 310 and low-temperature stress on cytochrome c release from winter wheat mitochondria are very similar.
Acknowledgment. The work has been performed, in part, with the support of the Russian Foundation of Basic Research (project 00-04-48093).
References.
S.V. Osipova, V.A. Trufanov, and T.A. Pshenichnikova.
Gluten content in common wheat is of great importance in breeding. A high content of storage protein is not a guarantee of high gluten quality, which depends substantially on the SS/SH status of these proteins (Bloksma 1975; Kretovich 1991). Wheat grains contain a specific system of enzymes belonging to the class of oxidoreductases that are responsible for the thiol-disulfide metabolism in proteins (Trufanov 1994; Trufanov et al. 1999). Studies of thiol:protein disulfide oxidoreductase (disulfide reductase, RED, EC 1.8.4.2) and thiol:oxygen oxidoreductase (thioloxidase, EC 1.8.3.2) activities in spring cultivars of wheat with different gluten quality have shown a correlation between activity and rheological properties of dough. The high genotypic variability of specific activity of thioloxidase and disulfide reductase was established in 18 common, spring wheat cultivars of different origins (Trufanov et al. 2000). Therefore, we were interested in investigating intervarietal substitution lines where a pair of chromosomes of a recipient cultivar are substituted for the homologues from a donor variety. In this paper, the results of study of disulfide reductase (RED) activity and some technological characteristics of grain in substitution lines involving chromosomes of homoeologous groups 1 and 6 of common wheat are presented. These chromosomes are known to have the genes for gluten formation (Wrigley and Shepherd 1973).
Materials and methods. Lines with substitutions for chromosomes 1A, 1B, 1D, 6A, 6B, and 6D were used (Maystrenko et al. 1998). The cultivar Diamant (DM), which has one of the highest grain-protein contents but poor technological properties, was the recipient, and the high-quality cultivar Novosibirskaya 67 (N67) was the donor parent. Disulfide reductase activity was determined according methods described earlier (Kichatinova et al. 1993; Trufanov 1994). The technological parameters studied are described in Trufanov et al. (2000). Figure 11 and Figure 12 show the average data of two independent replicates of experiment. The activity of disulfide reductase in each was determined three times in two biological and three analytical replicates. The data on specific activity and technological parameters are shown in percent of the recipient variety DM.
Results and discussion. According to modern concepts, the physical properties of the gluten-protein complex are determined considerably by the content of intra- and intermolecular SS-bonds in storage proteins. The formation, breaking, and isomerization are catalyzed by specific enzyme system of SS/SH metabolism. One of the key enzymes in this system, RED catalyzes the reaction that reduces the disulfide bonds and participates in formation of SH/SS status of storage proteins. Figure 11 and Figure 12 show that the 1A substitution line and double 1A, 6D substitution line have better technological properties, higher flour strength and extensibility, dough resistance, valorimeter number, loaf volume, and a lower dough dilution. Overall, the technological properties have been improved compared to the recipient DM in lines with these substitutions. The RED activity also was significantly lower than in the recipient in these lines. The activity of RED negatively correlates with water-absorbing capacity, dough resistance, and valorimeter number Table 1.
| Quality characteristic | RED |
|---|---|
| Flour water-absorbing capacity | - 0.759 * |
| Dough dilution | 0.415 |
| Dough resistance | - 0.674 * |
| Valorimeter | - 0.444 |
The positive correlation with dough dilution observed may be connected with the participation of this enzyme in breaking SS-bonds in the gluten structural matrix. Introducing the favorable Glu-A1a allele into a cultivar genotype by intervarietal substitution is known to improve quality (Mansur et al. 1990). Our data show another result of substitution for 1A chromosome; changes in RED activity with improvement of separate technological properties. We have not found any data concerning the chromosome localization of genes for RED in cereals. The significant reduction of RED activity in lines DM/N67 1A and DM/N67 1A,6D may indicate that these two chromosomes participate directly in the genetic control of this enzyme or in regulation of its activity.
References.
R.K. Salyaev, L.V. Dudareva, S.V. Lankevich, and V.M. Sumtsova.
The use of lasers has been growing steadily in the last 20 years, both in medical and biological research. The impact of laser irradiation on animal tissues and, particularly, on the human organism has been studied intensely (Devyatkov et al. 1987; Skobelkin 1997; Burlakova et al. 1998; Karu 1998; Rogatkin and Chernue 1999), whereas plants studies are relatively lacking. Plants, however, are better adjusted evolutionarily to the perception of light energy and its utilization in various physiological processes proven by the influence of irradiation on different biological units (Devyatkov et al. 1987; Skobelkin 1997; Karu et al. 1998; Bakeyeva et al. 1999; Grishko et al. 1999; Rogatkin and Chernue 1999). Numerous investigators confirm the stimulation of regenerative processes in animal and human tissues by laser irradiation (Burlakova et al. 1998). We assume a similar effect to be probable in plant tissues. The present work investigated the impact of low-power laser irradiation on morphogenesis and regeneration in the callus of wheat cultivars.
The following characteristics of cultivar tissue growth and development were determined: the number of morphogenic calli, secondary rhyzogenesis, and the number of regenerants. A helium-neon laser LG-75 with an irradiation wavelength of 632.8 nm and an intensity of 12 mV on the sample was used. The duration of the irridation was 5 min. Wheat calli of the variety Scala were used as plant material. Mature embryos with half of endosperm were used as explants. Callus induction was on a modified MS (Murashige/Scoog) medium with the addition of 2 % sucrose and 2 mg 2,4-D. Samples were irradiated on the second day after replanting on the first passage. The growth parameters were recorded for 100 calli in the test and for an equal number of control calli in three independent experiments. The number of morphogenic calli forming secondary differentiation zones, rhyzogenic zones, and the number of regenerants were calculated relative to the total amount of the samples under investigation. The reliability of differences between the average values compared was evaluated with a t-test. The dynamics of the formation of secondary differentiation zones in Scala calli during the course of the experiments is in Fig. 13.
The type of dependence identified confirms the earlier established, wavelike character of morphogenic process dynamics in cultivar callus (Kuzevanov et al. 1990), both for the samples subjected to irradiation and for the control samples. The number of morphogenic calli in the samples subjected to laser treatment was systematically higher than in the control samples, on the average of 20 %, up to the time (20-25th day of cultivation) when regenerant formation began. At that time, the number of morphogenic calli in the control and test samples were equal. By the beginning of active formation of regenerants and roots from these calli, both were more numerous in the samples subjected to irradiation than in control. Especially from a biotechnological view point, the data demonstrate the dynamics of plant-regenerant formation from calli (Fig. 14).
Over the observation period, the number of the first regenerants in the samples subjected to irradiation exceeded the corresponding values in the control samples. The number of regenerants in the test averaged 38 %, whereas in the control sample it did not exceed 25 %. Over the course of the three experiments, the calli subjected to irradiation were distinctly difference compared to the corresponding values in the control samples (P < 0.001).
Laser irradiation may impact not only intensity, but also space and time coherence and, possibly, primarily polarization. Because the laser bundle is linearly polarized and coherent, its impact on cell structures is most likely to be anisotropic in character (i.e., the phenomenon of light-induced membrane hyperpolarization is known (Tazawa et al. 1986)). Laser irradiation may induce morphogenic processes. Evidence of a particular mutual location of cells and cell structures (so called polarity) is one of the principal conditions for the beginning of regenerative processes (Poleovoi 1989).
Based on our results, we can conclude that low-intensity coherent irradiation without changing the general dynamics of differentiation processes in wheat cultivar tissues, produces a stimulating effect on these processes by a noticeable increase of secondary differentiation zone number, root seedlings, and number of regenerants. These data make possible the use laser as a factor that can influence morphogenesis in the tissues of higher plants.
References.
N.I. Rekoslavskaya, O.V. Yurieva, B.A. Shainyan (Irkutsk Institute of Chemistry, Siberian Branch of Russian Academy of Sciences, 664033, Irkutsk, Favorski str. 1, Russian Federation), T.V. Kopytina, and R.K. Salyaev.
Until recently, L-tryptophan (LTry) was presumed to be the main indole precursor of the key hormone of higher plants indolyl-3-acetic acid (IAA). In some plants, an enzyme system of IAA biosynthesis named D-tryptophan aminotransferase was reported to operate by using D-tryptophan (DTry) as a substrate (McQueen-Mason and Hamilton 1989). The formation of natural DTry is caused by the activity of another enzyme of tryptophan metabolism, a tryptophan racemase which converts LTry to DTry during several growing conditions (Law 1987). We assumed that when DTry appeared in plant tissues, it could be used simultaneously in IAA biosynthesis and in the formation of N-malonyl-D-tryptophan (MDTry) (Rekoslavskaya 1986), but not for synthesis of proteins. Rekoslavskaya et al. (1988) have shown that MDTry was accumulated in seeds and shoots of many plants during water lost.
Some researchers consider the process of formation of MDTry in plants to be an event of the IAA biosynthesis regulation system at the level of inactivation and reservation of the precursor. At the same time, the pathways of metabolism and the physiological role of this compound were not well studied.
The goal of the present study was to investigate activity of racemase in relation to the time course of germination and initial steps of growth and IAA biosynthesis. A second task was to assess configurations of MTry with respect to the biological activity of chemically synthesized enantiomers of MTry and to the growth of isolated embryos of T. aestivum and IAA level.
Materials and methods. The spring wheat cultivar Skala was used in this study. To determine the activity of tryptophan racemase, batches of 200 seedlings were harvested on the 3rd and 5th days after germination. Seedlings were ground in liquid nitrogen in a 0.66 M KH2PO4/Na2HPO4 buffer, pH 8.3, containing 20 µM pyridoxalphosphate, 1 mM Na EDTA, 4 mM MgCl2, 1 mM phenylmethylsulfonylfluoride, 20 % glycerol, and 0.1 % mercaptoethanol. The homogenate was centrifuged at 4 C and 10,000 X g for 20 min. The fraction of pellet enriched with etioplasts was used as an enzyme source. The reaction mixture containing 5 µmoles of DTry or LTry and enzyme preparation (15-20 mg of protein) was incubated 1 h at 37 C. The quantities of DTry and LTry were determined according to the methods of Nagata et al. (1988) using D-amino acid oxydase or L-amino acid oxydase in separate experiments.
The total tryptophan transaminase and dehydrogenase activity
was determined in etioplast fractions isolated according to methods
published earlier (McQueen-Mason and Hamilton 1989). The amount
of IAA was determined with HPLC with spectrofluorimetric detector
in extracts isolated and purified according to Rekoslavskaya (1986).
The amount of MTry was estimated after reaction with Ehrlich reagent
and the extinction was measured at 564 nm. The synthesis of MDTry
and MLTry was performed using the modified procedure reported
by Satoh and Esashi (1884) for the synthesis of malonyl derivative
of aminocyclopropane carboxylic acid.
Cultivation of embryos excised from dry seeds was on a modified
Norstog nutrient medium deprived of casein hydrolysate and amino
acids (Norstong 1973). Synthetic MLTry and MDTry were used as
auxin precursors added to the agar medium. The configuration of
the endogenous MTry was determined by chromatography on TLC Plates
C18-Silica on glass plates (Sigma, USA). Each experiment was repeated
twice at least. Data in tables are presented an average of two
or three analytical repeats with calculated standard deviation.
Results and discussion. All MTry found in 2-day-old wheat embryos was identified as MDTry at 30 nmol/g. The amount of IAA determined by HPLC was 3.970.34 nmol/g fresh weight on the second day after germination. After the 5th day, the amount of IAA diminished to 0.690.19 nmol/g fresh weight. The most intensive growth of etiolated coleoptiles was between the 3rd and 4th days after germination and initial growth of seedlings when the coleoptile usually elongates very fast up to 5-6 cm in length. The activity of tryptophan racemase was determined in 3-, 5-, and 7-day-old wheat seedlings germinated in darkness (Table 2).
| Days after germination | L -> D | D -> L |
|---|---|---|
| 3 | 2,239 | 344 |
| 5 | 313 | 210 |
| 7 | 41 | 112 |
Obviously there was found a correlation between IAA content and activity of conversion of LTry to DTry during initial 2-3 days of germination of wheat seeds. In order to compare the conversion of DTry and LTry to IAA, the total activity of tryptophan transaminase and dehydrogenase was evaluated (Table 3).
| Variant | Coleoptiles | Roots |
|---|---|---|
| LTry | 3,429 ± 30 | 4,546 ± 155 |
| DTry | 1,542 ± 44 | 603 ± 59 |
Only the potential activity, not the real activity, of the enzymes was measured in the reaction mixture. We believe that coleoptiles and roots are able to convert both stereoisomers to IAA in vitro, perhaps DTry, and its stored form MDTry, might be used for IAA biosynthesis during germination by supporting the energetic heterotrophic growth.
In previous studies (McQueen-Mason and Hamilton 1988; Rekoslavskaya 1986), the concept of the role of MTry in the regulation of IAA biosynthesis implied the participation of MDTry, because it was not assumed the accumulation of MLTry in plants. Therefore, we made a comparison of synthetic MLTry and MDTry on the growth in vitro of isolated wheat embryos.
The synthetic 200 µM MDTry stimulated the formation of roots in almost 90 % of the embryos grown on the agar medium during 20 days. Unlike MDTry, MLTry retarded the growth of all parts of the embryos at all concentrations. On the media with 100 and 200 µM MLTry, about 60 % to 80 % of the embryos, respectively, perished or remained unsprouted. The concentration of IAA in embryos grown on the MDTry-containing medium (120 ng/g) was considerably higher than that for the control and for growth on the MLTry-containing medium (10 and 40 ng/g, respectively).
The experimental material revealed a distinct correlation between the content of MDTry, IAA, and root-forming activity. The D enantiomer of MTry formed in the period of active root development in embryos of intact seedlings. We did not find MDTry in maturing wheat kernels. We concluded that racemization functions at the very early stages of germination and acts as a trigger stimulate fast, energetic germination to support auxin in the embryonic cells.
In native PAGE of purified D-tryptophan agarose-column enzyme preparations from etioplast fractions, we found the activity of tryptophan racemase as a band of 74 kD. While studying the kinetic parameters of wheat tryptophan racemase, we found that the Vmax and Km for etioplast racemase were 688 ± 26 nmol/h and 0.2 ± 0.1 mM for DTry and 2,588 ± 10 nmol/h and 0.6 ± 0.0 mM for LTry, respectively. We can assume that the shift of racemization in the L -> D direction was the consequence of the different stereospecificity of tryptophan racemase to DTry and to LTry.
Based on these data, we concluded that the formation of the endogenous MDTry in wheat embryos was probably one way of regulating IAA biosynthesis at the level of inactivation and reservation of the precursor. In this light, the chain of tryptophan transformations directed to IAA biosynthesis can be represented as follows.
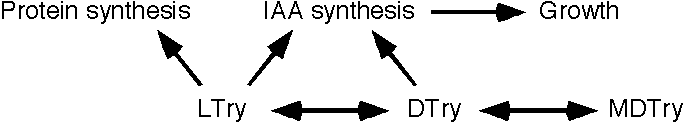
Thus, LTry can be used for the synthesis of IAA and protein simultaneously. On germination, under the conditions of activation of protein synthesis and increasing requirement in auxin, the system for forming and reserving the nonproteinogenous precursor of IAA DTry (DTry) acts, perhaps, as an additional regulatory component, independent on the main nitrogen metabolism that determines the IAA status and growth potential of embryos.
References.
V.A. Davydov.
By investigating monosomic wheat lines, numerous genetic effects
of various characters were revealed and their chromosomal localizations
of the corresponding genes established (Arbuzova and Maistrenko
1986; Goncharov 1992). Genes localized on chromosome 3A of winter
wheat affect stoma resistance (Bobo et al. 1992). The relationship
between the stomatal apparatus itself and individual chromosomes
remains unexplored, although it attracts a fair amount of attention
because it is associated with productivity and drought resistance
(Sherifi 1991; Zhuravleva 1992; Wang and Clarke 1993a; Tupitsyn
1995).
This study was on monosomic lines of common wheat variety Chinese Spring. The effect of the absence of individual chromosomes on quantitative characters of the stomatal apparatus (number of stomata/unit leaf area, distance between stomata in a row, distance between the rows, and length and width of stomata) were investigated. Monosomic lines of Chinese Spring for each chromosome of the A, B, and D genomes were grown under identical conditions in a growth chamber. Disomic Chinese Spring also was grown as a control.
Prints were taken from leaves by a procedure that involved smears of chloroform-dissolved polymetyl methacrylate (Davidov 1991). Taking into account the considerable variability of stomata indices even within a single leaf blade (Wang and Clarke 1993b), prints were taken from the lower and upper sides of the middle part of the preflag leaf at boot stage. The prints were examined microscopically and the results are given in Table 4 for the upper-leaf and Table 5 for the lower-leaf surfaces.
Number of stomata. Lines monosomic for chromosomes 2B, 1A, 6B, 2A, and 6A had the highest number of stomata on the upper-leaf surface and 5A, 1D, 4D, 3D, 2D, and 7D had the lowest number. On the lower side of the blade, lines monosomic for chromosomes 7B, 6B, 1A, 3B, and 2B had the highest number of stomata and 5A, 4D, 7D, 2D, 3D, 3B, 5D, and 2A had the lowest number (Table 4 and Table 5).
Generally, lines monosomic for A- and B-genome chromosomes differed little in the number of stomata on the upper-leaf surface (49 and 50 stomata/mm^2^), whereas in the D-genome monosomics this index was 43 stomata/mm2. The greatest value for the mean density of stomata on the lower-leaf surface was observed in monosomics for the B genome (28 stomata/mm^2^), slightly greater than for the A genome (24 stomata/mm^2^), with the least amount found in the D genome (18 stomata/mm^2^).
Distance between stomata. Despite the fact that in some lines the distance between stomata varied from 2-27 µm, the mean value of this index on the upper-leaf surface was fairly stable; it was very similar for all genomes and varied from 9.0-10.6 µm. The greatest mean distance between stomata in a row was observed in monosomic line 5A (15.5 µm) and the least was in monosomic 2A (6.4 µm). On the lower-leaf surface, the mean distance between stomata was approximately half of that of the upper surface, varying from 18-24 µm. However, in all lines, including the control, stomata were lacking in considerable areas, sometimes 100 µm or greater. On the upper surface, such gaps were observed only in lines monosomic for chromosomes 6A and 4B. Distance between the stomatal rows. On the upper-leaf surface, this index varied from 3-28 µm and averaged over the groups varied from 10.9-12.8 µm. On the lower surface, this character was more variable (5-65 µm), averaging from 14.9-17.7 µm.
Stoma length and width. On the upper-leaf surface, stoma length varied from 3.71 (monosomic 7A) to 5.44 µm (monosomic 3D). The values of this index averaged over the groups were very close, from 4.49-4.83 µm. Stoma width varied from 2.19 µm (monosomic 6B) to 3.02 µm (monosomic 4B). The value of this index averaged over the groups was less variable than that of width, from 2.54-2.67 µm. On the lower-leaf surface, the shortest stomata were found in the line monosomic for 7A (3.79 µm) and the longest was in monosomic line 4A (5.62 µm).Stoma width on the lower leaf side varied from 2.08 µm (monosomic 6D) to 3.25 µm (monosomic 3B). The values averaged over the genome groups were similar, ranging from 2.51-2.67 µm. Although the ratios between the length and width of stomata in individual lines varied from 1.39-2.44 (lower-leaf surface, monosomic lines 7A and 4A), their mean values were 1.70-1.89.
Linear sizes of stomata were affected most by the absence of chromosome 7A. The mean stoma length in this line was less than that of the control by 21.9 and 22.5 % on the upper- and lower-leaf surfaces, respectively. The absence of chromosome 6D genes also affected the stoma length significantly. In this line, the mean stoma lengths were 14.1 and 9.0 % less than in the control on the upper and lower surfaces, respectively. By contrast, in momosomic 4A, an increase in mean stoma length by 17 % on the upper surface and 15% on the lower surface with respect to the control were observed.
Monosomy also can have the opposite effect, where the absence of a given chromosome may increase or decrease the value of a character in some cases (Tsil'ke and Tsil'ke 1973). The coefficient of variation is an important index of a character that measures the degree of variability. Distance between stomata on the lower-leaf surface had the greatest coefficient of variation, from 50.0 % in monosomic 5A to 154.5 % in monosomic 2B, which was explained by irregular positions of stomata within the rows. On the upper surface, the coefficient of variation of this index was much lower. Only in lines monosomic for chromosomes 6A (143.7 %) and 4B (139.4 %) was an irregular stoma distribution observe and did not exceed 60 % in the other lines. The coefficient of variation for distance between stoma rows did not exceed 50 % on either leaf surface. The number of stomata had an even lower coefficient of variation, below 40 % on the lower surface and below 15 % on the upper. The linear size of stomata were the least variable. The coefficient of variations were approximately equal on both sides and usually did not exceed 10 %.
Chromosome dosage, which effects gene dosage in various monosomic lines of Chinese Spring wheat, may cause considerable changes in the features of the stomatal apparatus. Absence of one dose of genes of the critical chromosome 5A exerted the greatest effect on the number of stomata and caused a decrease in their density by almost one-third on the upper-leaf surface and by more than a half on the lower, when compared with disomic Chinese Spring plants. The lack of some of the other chromosomes produced lesser effects, but also effected both the number of stomata and their linear sizes.
Acknowledgment. The author is grateful to G.G. Vershinina for her kind permission to take prints from the monosomic lines.
References.
A.V. Permyakov, S.L. Didenko, and V.A. Trufanov.
Lipoxygenases (Lpx, linoleate:oxygen oxidoreductase, EC 1.13.11.12) occur in plants as groups of enzymes catalyzing dioxidation of unsaturated fatty acids with forming superoxide radicals that may in vivo oxidize SH-groups of wheat storage proteins (Grechkin 1998). Best studied are soy seed lipoxygenases, where they amount to 2 % of protein and are represented by three isoforms (Axelrod et al. 1981). Durum wheat caryopses also contain three molecular forms of this enzyme with a molecular mass approximately of 100 kD (Hsieh and McDonald 1984). Thus, investigators are interested in lipoxygenase primarily because of its role in the in vivo formation of oxide radicals that may oxidize SH-groups of wheat storage proteins with the formation of inter- and intramolecular disulfide bonds stabilizing gluten protein complex. The influence of each lipoxygenase isoform is connected with SH-groups oxidation of storage proteins and improvement of certain gluten quality parameters (Shiiba et al. 1991). In this respect, we were interested in investigating the combination of molecular forms of lipoxygenase from various cultivars grains of wheat.
Materials and methods. Scala, Tulunskaja 12, Rollo, and Drott of cultivars grains of wheat were chosen for our investigations. The soluble, enzymatically active protein fraction of the wheat grain was extracted with a Tris buffer, pH 7.5, containing 5 mM EDTA, from standard ground flour in the proportion 1:2 (weight:volume). Subunit composition of the acquired fractions separated in SDS-PAGE according to the method of Laemmli (Laemmli 1970). Native salt-soluble proteins of the wheat grains of various cultivars were separated according to the method of Davis (1964) at the basic pH. Molecular forms of Lpx were identified immediately on gel slabs by specific coloring with sodium linoleate and iodine-starch reagent (Heydeck and Scewe 1985).
Results and discussion. Structural genes encoding lipoxygenase synthesis are known to be localized in chromosomes of homoeologous groups 4 and 5 (Li et al. 1999). The study of lipoxygenase activity in the grain of soft wheat varieties Saratovskaja 29 and Janetzkis Probat and their with intervarietal substitution lines of individual chromosome pairs 4A, 4B, 4D, 5A, 5B, and 5D indicated reliable differences in the effects of these chromosomes by the level of lipoxygenase activity in the recipient variety Saratovskaja 29 (Didenko et al. 2001). The results indicated genotypic differences in the expression of lipoxygenase structural genes and the important role in the demonstration of gene-regulator enzyme activity.
To establish variety-specificity in the level of lipoxygenase activity, we compared electrophoretic spectra of salt-soluble protein fraction of the caryopses of four varieties of spring soft wheat of different origin (Fig. 15). Specific coloring of protein spectra acquired by native PAGE-electrophoresis and iodine-starch reagents (Heydeck and Schewe 1985) allowed the identification from each variety of all three molecular forms of lipoxygenase with relative electrophoretic mobility 0.37, 0.32, and 0.24 marked Lpx-1, Lpx-2, and Lpx-3, respectively. In native state, the Lpx-1 and Lpx-3 isoforms significantly differ by their molecular surface charge and by relative electrophoretic mobility.
Subunit composition of salt-soluble proteins of individual caryopses of the four wheat varieties with electrophoretic fractioning in SDS-PAGE proved to be very heterogeneous (Fig. 16). Nevertheless, subunit spectra for all the wheat varieties studied were similar and contained relatively high molecular polypeptides with molecular weights of 115, 105, 94, 85, and 75 kD.
The presence of three identical isoforms of native lipoxygenase and protein subunits with identical molecular weight in the grain of genotypically different wheat varieties allows us to infer similarity in the expression of genes localized in chromosomes of homoeologous groups 4 and 5.
The results are to some extent contradictory with the data of Shiiba et al. (1991), who reported the presence in wheat caryopses of three lipoxygenase isoforms (L-1, L-2, and L-3) differing in the value of surface charge and affinity to various ion-exchangers, but characterized by the same mobility after SDS-PAGE electrophoresis (rf = 0.28) and a molecular weight of about 110 kD.
References.
L.V. Pomazkina and L.G. Kotova.
Technologic pollution is known to affect field crop metabolism and harvest. Plant response to the accumulation of pollutants in the soil depends on their toxicity, soil properties, and species of field crop (Kabata-Pendias and Pendias 1986; Il'yin 1991; Pomazkina et al. 1999a). This research is highly topical due to the pollution of arable soils in the Baikal Region by industrial exhaust from Russia's largest aluminum plants. Fluoride compounds classed as highly toxic prevail in the exhaust and their impact on mineral nutrition and productivity of spring wheat has not been studied sufficiently.
We hope to identify the impact of technologic pollution by water-soluble fluorides of gray forest soil on the productivity and mineral nutrition of spring wheat and fluorine accumulation. Greenhouse experiments were made on fluoride-polluted and unpolluted soils with similar properties. The humus content in unpolluted soil is 2.2 %, total N is 0.15, salt is 5.7, and base exchange is 24.4 mg-equiv/100 g. In polluted soil the humus content is 2.5 %, total N is 0.13 %, salt is 5.6, and base exchange is 24.4 mg-equiv/100 g. These soils have a very low content of dynamic macroelements. The content of water-soluble fluorides in unpolluted soil correspond to the regional background, 5 mg/g of soil. In soil zones located in the area of local pollution by the aluminum plant, the water-soluble fluroride content is 60 mg/kg. The high level of pollution (220 mg/kg) was modeled by the additional introduction of NaF into the soil.
Experiments were conducted in a phytotron in the pots with 4 kg of soil. During the sowing season until the emergence of sprouts, air temperature was maintained at the level of 25 C and during the vegetative season, the daytime temperature was 20 C and the night temperature was 18 C. During the 16-h light period, the degree of light amounted to 10,000 lux. Soil humidity was maintained by daily irrigation with distilled water and was calculated as 60 % of total moisture capacity. Experiments included a control (no fertilizer), an NPK treatment, and an NPK + NaF treatment. We used chemically pure salts of Ca(H2PO4)2, KCl, and NH4NO3, which were introduced during filling of the pots. The dose of N, P, and K was 0.1 mg primary nutrient/kg of soil. The spring wheat Skala was planted as germinated sprouts at a rate of 14 plants/pot. The plants were harvested in the blooming phase. The experiments were replicated three times.
Total nitrogen in the plants was determined by the procedure, protein nitrogen with trichloroacetic acid, phosphorus by Alen's method with amidol, and potassium by flame emission photometer. Fluorine content was analyzed using arsenase by spectrophotocolorimetrical methods. Statistical data was processed by Microsoft Excel 2000.
Wheat productivity was more dependent on the amount of macroelements in the soils than by pollution by water-soluble fluorides (Table 6). In unpolluted and polluted soils, the surface mass of wheat was low in the control plants and high in the NPK treatment. The efficiency of fertilizers was highest in unpolluted soil. In the soils with NaF, the surface mass was 40 % lower when compared to the background. This reduction may be connected not only with fluorine phytotoxicity, but with an increase in sodium content in the soil.
| Soil type | F water-soluble (mg/kg) | Treatment | Biomass (g/pot) | Addition to control (g/pot) | F, mg/kg of dry matter | |
|---|---|---|---|---|---|---|
| Surface | Roots | |||||
|
|
|
Control | 4.2 | --- | 55 | 56 |
| NPK | 13.9 | 9.6 | 56 | 73 | ||
| LSD05 | 0.4 | 11.0 | 20 | |||
|
|
|
Control | 4.0 | --- | 88 | 110 |
| NPK | 10.0 | 6.9 | 63 | 80 | ||
|
|
|
NPK+ NaF | 6.5 | 2.5 | 64 | 1,170 |
| LSD05 | 2.1 | 15.0 | 25 | |||
Fluorine content in the surface mass and roots of wheat grown
in polluted soil was higher, particularly when no fertilizers
were used. The comparatively low accumulation in plants supplied
with NPK was apparently conditioned by a dilution effect due to
high productivity. High fluorine content with NaF treatment was
noticed only in the roots, which are characterized by their barrier
function. Fluorine accumulation in the roots is known to produce
a negative effect on plant metabolism (Vlasyuk 1969).
| Soil | F water-soluble (mg/kg) | Treatment | Surface | F, mg/kg of dry matter | |||
|---|---|---|---|---|---|---|---|
| N:P:K | N, mg/g of dry matter | Nonprotein N % of total | Surface | Roots | |||
|
|
|
Control | 40:11:49 | 15.8 | 31 | 35:9:56 | 8.8 |
| NPK | 52:9:39 | 23.3 | 39 | 62:7:31 | 15.8 | ||
| LSD05 | 2.5 | 3.2 | |||||
|
|
|
Control | 40:14:46 | 19.3 | 28 | 38:13:49 | 10.8 |
| NPK | 59:9:32 | 26.9 | 44 | 68:5:27 | 19.5 | ||
| 220 | NPK + NaF | 63:8:29 | 31.7 | 52 | 78:6:16 | 22.0 | |
| LSD05 | 3.3 | 3.0 | |||||
The absorption of certain macroelements by wheat on fluoride-polluted soils is demonstrated their proportion in the tissues (Table 7). During anthesis, the optimum N:P:K content in spring wheat is considered to be approximately 50-57:7-9:36-43 (Tserling 1990). NPK treated plants showed approximately the same parameters in unpolluted soil. Tests on both soils showed a decrease in nitrogen, which is partially due to with its lack in the soil. In polluted soil in plants with an NPK treatment, the nitrogen share was higher, particularly in the variant with NaF (63 %). Similar changes in N:P:K proportions were observed in wheat roots.
The negative effect of soil pollution by fluorides also was observed in the increase of nitrogen required for the formation of 1 gram of dry matter in the above-ground parts and roots of wheat, particularly in the NaF-treated soil. The increase in the nitrogen content of these plants may be considered a nonspecific reaction caused by the toxicant and corresponds to an increase in nitrogen consumption by wheat in stress conditions (Al'tergot et al. 1974; Pomazkina et al. 1999a). Intensive nitrogen absorption by plants could be a consequence of increases in soil-nitrogen mineralization under technologic pollution of soils (Pomazkina et al. 1999b, 1999c).
An increase in the nonprotein-nitrogen fraction in wheat tissues also indicates nitrogen exchange on fluoride-polluted soils. For example, in the above-ground parts of the plants in the NPK treatment on polluted soil, nonprotein nitrogen amounted to 44 % of total nitrogen. With the NaF treatment, nonprotein nitrogen was 52 %, in contrast to 39 % in the plants on unpolluted soil. This decrease may be due to lower synthesis processes because of higher fluorine content in the roots.
The negative impact of technologic pollution of soils by fluorides was demonstrated in the disturbance of mineral nutrition and metabolism of spring wheat. We observed changes in the proportion of N:P:K in the tissues, largely because of increases in nitrogen. The increase in nonprotein nitrogen in the above-ground plant parts demonstrates an imbalance of synthesis-decomposition processes. The most significant changes responsible for the decrease in wheat productivity were identified when modeling high pollution levels. The disturbances revealed corresponded to fluorine accumulation in the roots.
References.