Search for resistance against
Blumeria graminis f.sp. hordei in barley landraces from
M. J. Y. Shtaya1, J. C. Sillero2
and D. Rubiales1,
1 Institute of Sustainable
Agriculture, CSIC, Apdo. 4084, 14080
2 CIFA,
Abstract
A collection of 111 barley
landraces from the
Keywords Barley, Blumeria
graminis f.sp. hordei, Hordeum vulgare, landraces, powdery
mildew.
Introduction
Powdery mildew
caused by Blumeria graminis f.sp. hordei is one of the most
important highly variable foliar diseases on barley (Hordeum vulgare),
which causes severe losses and quality reduction especially for the production
of malting barley (Balkema-Boomstra
and Mastebroek 1995; Czembor 2001). Yield losses due to powdery mildew
can reach 30%. Early infection reduces tillering, while late infection of the
upper leaves and of the spike can heavily reduce grain yield (Scott and
Griffiths 1980).
Powdery mildew on
barley can be controlled by the use of fungicides and of resistant cultivars. Breeding
for resistance is a cheap alternative approach to reduce the loss in yield
caused by powdery mildew. Many single resistance genes to powdery mildew have
been identified and introduced into current barley varieties. Most of these
genes originated from barley landraces and from wild relatives (Xu and Kasha
1992; Jahoor and Fischbeck 1993; Jørgensen and Jensen 1997; Czembor and Czembor
2000). However, these race-specific genes are not durable due to rapid changes
in virulence in the pathogen (Dreiseitl and Jorgensen 2000; Dreiseitl and Bockelman
2003), what reinforce the need of searching new sources of resistance.
The objective of the
present study was to determine levels of resistance to powdery mildew present
in a collection of barley landraces from the
Materials and methods
Plant material
Seed samples of 111 H.
vulgare landraces from the Fertile Crescent (Table 1) were kindly provided
by the International Centre for Agricultural Research in the Dry Areas -
ICARDA,
Table 1 Origin and source
of the barley landraces used in this study.
|
Origin |
Number of
accessions |
Source |
|
|
4 |
USDA |
|
|
29 |
ICARDA + USDA |
|
|
15 |
ICARDA |
|
|
23 |
ICARDA + USDA |
|
|
40 |
ICARDA |
Inoculum
Isolate CO-02 of B.
graminis f. sp. hordei (virulence / avirulence factors Mla8,a1,a7,a9,a10,a12,a22,a23,k,p,g,La,h/a3,a6,a14,a13,at,o5)
collected at Cordoba, Spain was used in the experiment. The isolate was
maintained and increased on young seedlings of the cultivar Vada.
Testing procedure
About 10-15 seeds per accession were sown in 7x7x11 cm
boxes. Eleven days after sowing when the primary leaf was fully expanded, 50mm
of a central leaf segment was excised from each seedling, and placed adaxial
surface up in a square Petri dish (11 x 11 cm) filled with 0.6% agar and 125
ppm Benzimidazole. In each Petri dish, segments of six accessions were randomly
fixed, (2-4 segments per line), in three replicates. One day before inoculum
was required, heavily infected plants were shaken to remove ageing conidia to
ensure a supply of vigorous young spores. Inoculation was made by blowing
spores from the infected plants over the leaf segments using a settling tower.
A glass slide was placed in the settling tower to monitor inoculum density,
which was adjusted to give approximately 20 conidia mm-2 (Haugaard
et al., 2002). After inoculation, Petri dishes were transferred to a growth
chamber at 18-20 ºC and incubated in darkness for 12 h. They were then
transferred to a growth chamber with fluorescent lighting (12 h light / 12 h
dark) and 18-20 °C (Edwards, 1993).
Macroscopic observation
Infection type
(IT) was recorded five days after inoculation, following the 0-4 scale of
Moseman (1965) where: 0 = no visible signs of infection; 1 = brown necrotic
lesions with little or no mycelial development; 2 = some necrosis and chlorosis
with slight to moderate mycelial development; 3 = chlorosis with moderate
mycelial development; and 4 = abundant mycelial development with little of no necrosis
or chlorosis. Infection frequency (IF) was calculated as number of powdery
mildew colonies per cm2.
Table
2. Infection
type (IT) and infection frequency (IF), of 19 single-plant barley accessions against
powdery mildew
|
Accessions* |
IT |
IF |
|
IG29088-R |
0(4)
|
4 |
|
IG29088-S |
4 |
39 |
|
IG32722-R |
2
|
21 |
|
IG32722-S |
4 |
53 |
|
IG32733-R |
1 |
0 |
|
IG32733-S |
4 |
48 |
|
IG32799-R |
2
|
29 |
|
IG32799-S |
3-4 |
61 |
|
IG33094-R |
2
|
12 |
|
IG33094-S |
4 |
48 |
|
IG35223-R |
1 |
0 |
|
IG35223-S |
3-4 |
36 |
|
IG110851-R |
1
|
0 |
|
IG110851-S |
3-4 |
45 |
|
IG110857-R |
2
|
15 |
|
IG110857-S |
4 |
44 |
|
IG110887-R |
2
|
12 |
|
IG110887-S |
4 |
32 |
|
IG110895-R |
0(4) |
5 |
|
IG110895-S |
4 |
43 |
|
IG110899-R |
1 |
0 |
|
IG110899-S |
4 |
46 |
|
IG110905-R |
1
|
0 |
|
IG110905-S |
4 |
40 |
|
IG110906-R |
1 |
0 |
|
IG110906-S |
4 |
47 |
|
IG110909-R |
1
|
0 |
|
IG110909-S |
4 |
55 |
|
IG115774-R |
0 |
0 |
|
IG115774-S |
4 |
45 |
|
IG125770-R |
2
|
0 |
|
IG125770-S |
4 |
41 |
|
IG125773-R |
2
|
15 |
|
IG125773-S |
3-4 |
35 |
|
PI 223142-R |
2
|
15 |
|
PI 223142-S |
4 |
44 |
|
PI 253574-R |
2
|
10 |
|
PI 253574-S |
3-4 |
46 |
|
IG27377 |
4 |
39 |
|
IG32580 |
4 |
37 |
|
IG115778 |
4 |
36 |
|
IG125766 |
4 |
36 |
|
IG125767 |
4 |
37 |
|
IG125768 |
4 |
37 |
|
IG125778 |
4 |
35 |
|
IG128167 |
4 |
39 |
|
CIho2623 |
4 |
32 |
|
PI 223145 |
4 |
33 |
|
Vada |
4 |
69 |
* In
accessions in which segregation for IT was observed, individual plants with low
IT were recorded separately (accession-R), those with high IT (accession-S).
Results and Discussion
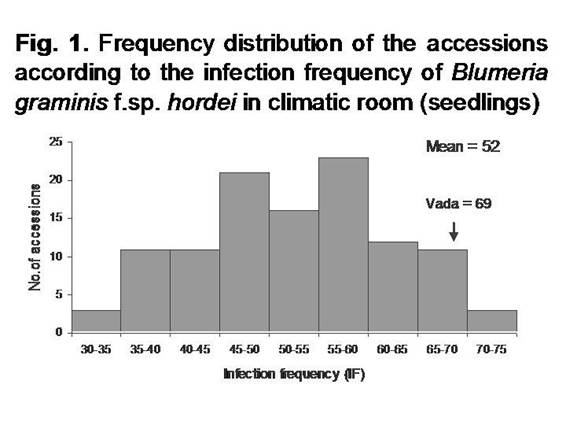
The susceptible
check Vada showed IF of 69 colonies per cm2 (Fig. 1). High
susceptibility was common in the collection with average IF of 53 colonies/cm2.
Most of the accessions (83%) displayed compatible interaction (IT 3-4). In
remaining 17% of the collection (19 accessions), segregation for IT was
observed, with individual plants showing low IT (Table 1). Fourteen accessions (13% of the collection)
showed low IF (IF < 40 colonies per cm2) in spite of a high IT
(Fig. 1 and Table 2).
Barley breeders
are seeking gene pools from which new genes can be introduced into existing
cultivars in order to improve their resistance to powdery mildew. Barley
landraces, especially those originated from centres of origin for cultivated
barley, constitute such a gene pool (Jørgensen and Jensen 1997; Czembor and
Johnston 1999; Czembor and Czembor 2000). This study showed that barley
landraces from
Seedling test does
not necessary predict adult plant resistance and field performance of the
selected resistant accessions, but are considered effective and sufficient to
postulate race-specific resistance genes and the identification of levels of
partial resistance (Dreiseitl and Jørgensen 2000; Backes et al., 1996). Some of
the accessions of this study showed low IF in spite of a high IT (Table 2).
They can be used as an additional source for partial resistance to powdery
mildew.
From the
collection, 19 single-plant lines with low IT were derived and grown in the
greenhouse to obtain seeds. The presence of reaction types 0, 0(4), 1 and
Acknowledgment
We thank the International Centre for Agricultural Research in the Dry Areas
(ICARDA) and United States Department of Agriculture (USDA) for providing the
seed samples used in this study. The Spanish Agency for International
Cooperation (AECI) and CICYT project
AGL 2005-01781 for financial support.
References
Backes,
G., G., Schwaez, G. Wenzel and A. Jahoor. 1996. Comparison between QTL analysis
of powdery mildew resistance in barley based on detached primary leaves and on
field data. Plant Breeding 115: 419-421.
Balkema-Boomstra,
A. G., and H. D. Mastebroek. 1995. Effect of powdery mildew (Erysiphe
graminis f.sp. hordei) on photosynthesis and grain yield partially
resistance genotypes of spring barley (Hordeum vulgare L.). Plant
Breeding 114: 126-130.
Czembor,
J. H. 2001. Sources of resistance to powdery mildew (Blumeria graminis
f.sp. hordei) in Moroccan barley land races. Canadian Journal of Plant
Pathology 23:260-269.
Czembor,
J. H., and H. J. Czembor. 2000. Powdery mildew resistance in selections from
Moroccan barley landraces. Phytoparasitica 28: 65-78.
Czembor,
J. H., and M. R. Johnston. 1999. Resistance to powdery mildew in selections
from Tunisian landraces of barley. Plant Breeding 118: 503-509.
Dreiseitl,
A., and H. E. Bockelman. 2003. Sources of powdery mildew resistance in a wild
barley collection. Genetic Resources and Crop Evolution 50: 345-350.
Dreiseitl,
A., and J. H. Jørgensen. 2000. Powdery mildew resistance in Czech and Slovak
barley cultivars. Plant Breeding 119: 203-209.
Edwards,
H. H. 1993. Light affects the formation and development of primary haustoria of
Erysiphe graminis hordei in leaf epidermal cells of Hordeum vulgare.
Physiological and Molecular Plant Pathology 42: 299 – 308.
Haugaard,
H., D. B. Collinge, and M. F. Lyngkjær. 2002. Mechanisms involved in control of
Blumeria graminis f. sp. hordei in barley treated with mycelial
extracts from cultured fungi. Plant Pathology 51: 612 – 620.
Jahoor,
A., and G. Fischbeck. 1993. Identification of new genes for mildew resistance
of barley at the Mla locus in lines derived from Hordeum spontaneum. Plant
Breeding 110: 116 – 122.
Jørgensen,
J. H., and H. P. Jensen. 1997. Powdery mildew resistance in barley landrace
material. I. Screening for resistance. Euphytica 97: 227-233.
Moseman,
J. G. 1965. Genetic studies with cultures of Erysiphe graminis f. sp. hordei
virulent on Hordeum spontaneum. Trans. Brit. Mycol. Soc. 48: 479 – 489.
Scott,
S. W., and
Van
Leur, J. A. G., S. Ceccarrelli, and S. Grando. 1989. Diversity for disease
resistance in barley landraces from
Xu,
J., and K. J. Kasha. 1992. Transfer of a dominant gene for powdery mildew
resistance and DNA from Hordeum bulbosum into cultivated barley (H.
vulgare). Theoretical and Applied Genetics 84: 771 – 777.